
Concept explainers
(a)
Interpretation: To identify whether the statement “cysteine and serine are standard amino acids that contain the element sulfur” concerning sulfur-containing amino acids is true or false.
Concept introduction: Amino acids are the
There are 20 amino acids present in nature. These are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, asparagines, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
(b)
Interpretation: To identify whether the statement “the molecule
Concept introduction: There are 20 amino acids present in nature. These are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, asparagines, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
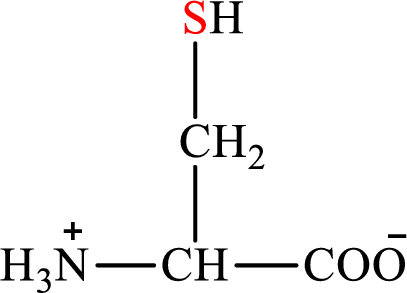
Cysteine gives pyruvate as the degradation product. The degradation of cysteine is a two-step process.
(c)
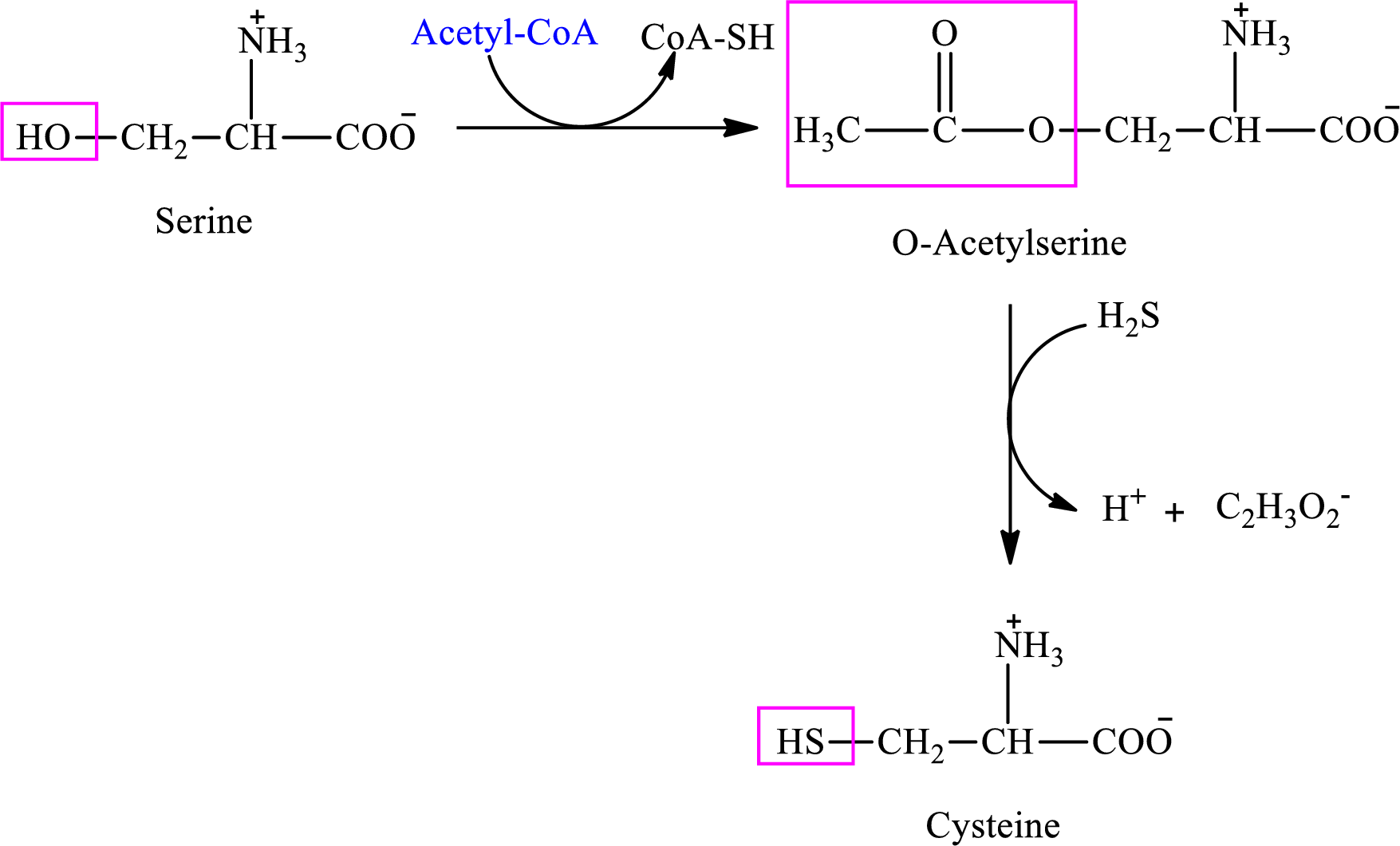
Interpretation: To identify whether the statement “the first step in the synthesis of cysteine from serine is an activation step” concerning sulfur-containing amino acids is true or false.
Concept introduction: There are 20 amino acids present in nature. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
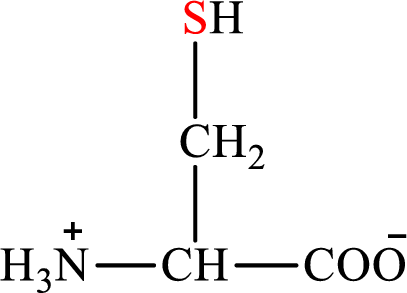
Cysteine is biosynthesized from serine that involves two steps.
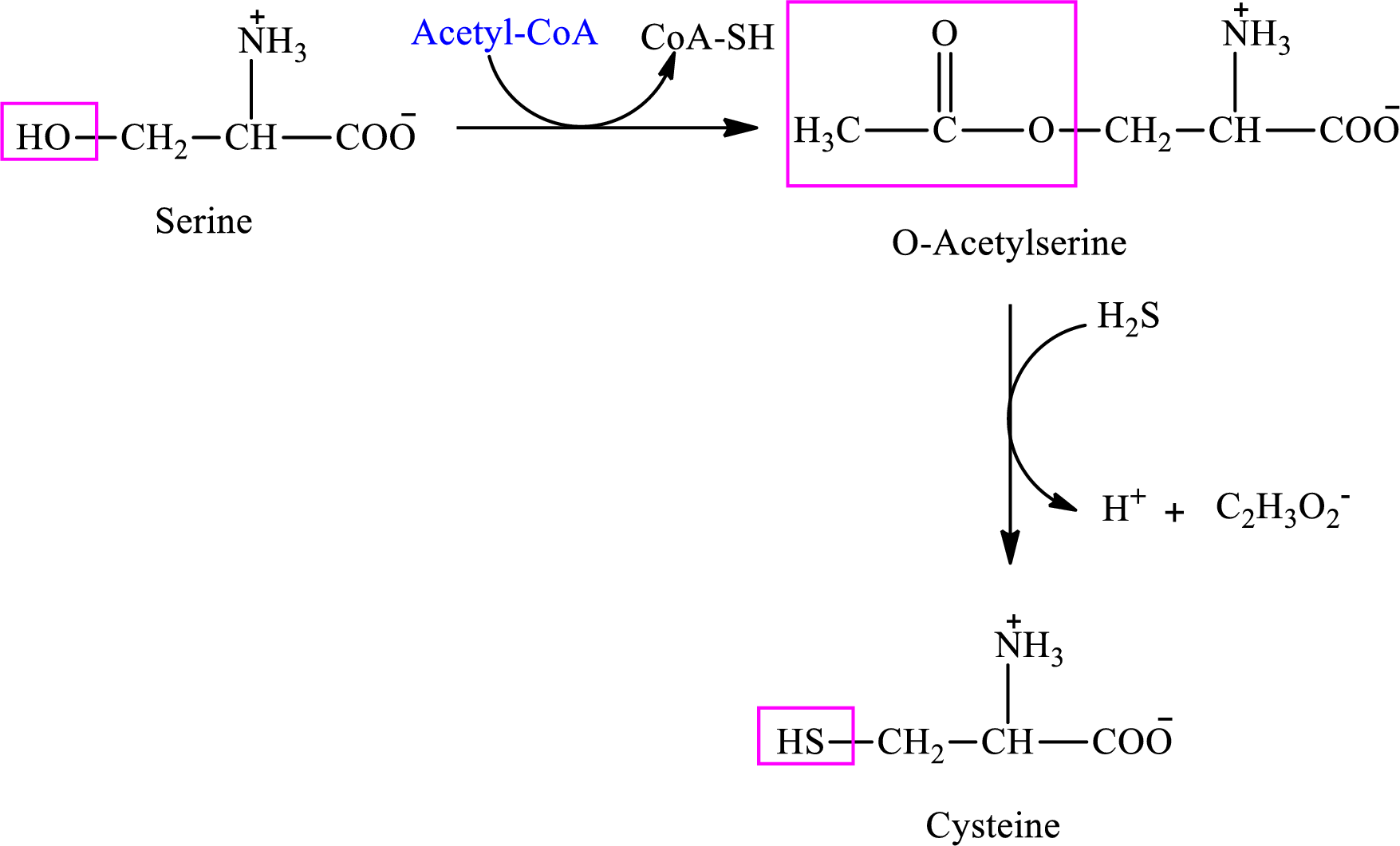
(d)
Interpretation: To identify whether the statement “in the synthesis of cysteine from serine it is
Concept introduction: There are 20 amino acids present in nature. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
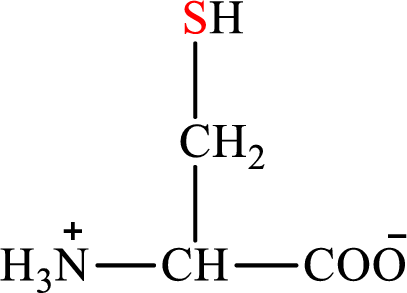
Cysteine is biosynthesized from serine that involves two steps.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





