
Concept explainers
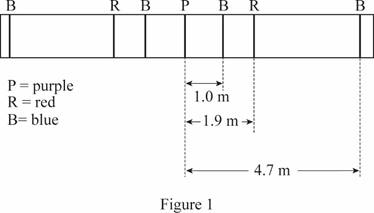
The sketch of the grating spectra with its labels.
Answer to Problem 86P
The diffraction spectra is given in figure 1.
Explanation of Solution
The wavelength of the red light is
Write the expression for slit separation
Here,
Substitute
Write the expression for diffraction maxima
Here,
Rearrange for
Write the expression for
Here,
Rearrange for
Substitute (II) in (III)
Substitute
Substitute
Substitute
Substitute
Substitute
The blue light and red light overlap for the central fringe and hence it appears purple.
The grating spectra drawn using the above information is given below:
Want to see more full solutions like this?
Chapter 25 Solutions
Physics
- A 15.8-mW laser puts out a narrow beam 2.0 mm in diameter.Suppose that the beam is in free space. What is the rms value of E in the beam? What isthe rms value of B in the beam?Prob. 28, page 834. Ans: Erms= 1380 V/m, Brms =4.59×10-6 Tarrow_forwardA 4.5 cm tall object is placed 26 cm in front of a sphericalmirror. It is desired to produce a virtual image that is upright and 3.5 cm tall.(a) What type of mirror should be used, convex, or concave?(b) Where is the image located?(c) What is the focal length of the mirror?(d) What is the radius of curvature of the mirror?Prob. 25, page 861. Ans: (a) convex, (b) di= -20.2 cm, i.e. 20.2 cm behind the mirror,(c) f= -90.55 cm, (d) r= -181.1 cm.arrow_forwardA series RCL circuit contains an inductor with inductance L=3.32 mH, and a generator whose rms voltage is 11.2 V. At a resonant frequencyof 1.25 kHz the average power delivered to the circuit is 26.9 W.(a) Find the value of the capacitance.(b) Find the value of the resistance.(c) What is the power factor of this circuit?Ans: C=4.89 μF, R=4.66 Ω, 1.arrow_forward
- A group of particles is traveling in a magnetic field of unknown magnitude and direction. You observe that a proton moving at 1.70 km/s in the +x-direction experiences a force of 2.06×10−16 N in the +y-direction, and an electron moving at 4.40 km/s in the −z-direction experiences a force of 8.10×10−16 N in the +y-direction. What is the magnitude of the magnetic force on an electron moving in the −y-direction at 3.70 km/s ? What is the direction of this the magnetic force? (in the xz-plane)arrow_forwardA particle with a charge of −5.20 nC is moving in a uniform magnetic field of B =−( 1.22 T )k^. The magnetic force on the particle is measured to be F=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the x component of the velocity of the particle.arrow_forwardIs it possible for average velocity to be negative?a. Yes, in cases when the net displacement is negative.b. Yes, if the body keeps changing its direction during motion.c. No, average velocity describes only magnitude and not the direction of motion.d. No, average velocity describes only the magnitude in the positive direction of motion.arrow_forward
- Tutorial Exercise An air-filled spherical capacitor is constructed with an inner-shell radius of 6.95 cm and an outer-shell radius of 14.5 cm. (a) Calculate the capacitance of the device. (b) What potential difference between the spheres results in a 4.00-μC charge on the capacitor? Part 1 of 4 - Conceptualize Since the separation between the inner and outer shells is much larger than a typical electronic capacitor with separation on the order of 0.1 mm and capacitance in the microfarad range, we expect the capacitance of this spherical configuration to be on the order of picofarads. The potential difference should be sufficiently low to avoid sparking through the air that separates the shells. Part 2 of 4 - Categorize We will calculate the capacitance from the equation for a spherical shell capacitor. We will then calculate the voltage found from Q = CAV.arrow_forwardI need help figuring out how to do part 2 with the information given in part 1 and putting it in to the simulation. ( trying to match the velocity graph from the paper onto the simulation to find the applied force graph) Using this simulation https://phet.colorado.edu/sims/cheerpj/forces-1d/latest/forces-1d.html?simulation=forces-1d.arrow_forwardI need help running the simulation to get the result needed.arrow_forward
- How can I remember this Formula: p = m × v where m is in kg and v in Meter per second in the best way?arrow_forwardHow can I remember the Formula for the impulsearrow_forwardA Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius г (see figure below) with a gas filling the space between the electrodes. Assume that the internal diameter of a Geiger-Mueller tube is 3.00 cm and that the wire along the axis has a diameter of 0.190 mm. The dielectric strength of the gas between the central wire and the cylinder is 1.15 × 106 V/m. Use the equation 2πrlE = 9in to calculate the maximum potential difference that can be applied between the wire and the cylinder before breakdown occurs in the gas. V Anode Cathodearrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





