
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25, Problem 25.64P
Tertiary
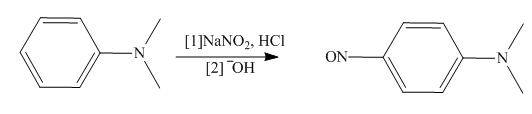
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
With this, please answer the following questions:
1.) draw the structure of the electrophilic intermediate in this reaction.
2.) what is the role of the AlCl3 in the reaction
3.) write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structures
Consider the data below to answer the following questions.
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones
and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic
acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a
cyanohydrin is treated with aqueous base the original carbonyl compound is isolated.
HO
H
HCEN
H-3-
HO'
NaOH
HO
cyanohychin
a.
a nucleophilic substitution
b.
an electrophilic addition
C10
OH
CH-COOH
A. The reaction of an aldehyde with hydrogen cyanide is an example of
+ NaCN + H₂O
reaction.
H-
C.
an electrophilic substitution
d. a nucleophilic addition
B. Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.
Refer to the data below to answer the following questions:
The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used
therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following
amino acids:
Ala, Arg, His, Pro, Sar, Tyr, Val, Val
A. Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine.
B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus.
Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments:
Tyr-Val-His
Sar-Arg-Val
His-Pro-Ala
Val-Tyr-Val
Arg-Val-Tyr
What is the structure of saralasin?
Chapter 25 Solutions
ORGANIC CHEMISTRY
Ch. 25 - Prob. 25.1PCh. 25 - Prob. 25.2PCh. 25 - Prob. 25.3PCh. 25 - Prob. 25.4PCh. 25 - Prob. 25.5PCh. 25 - Prob. 25.6PCh. 25 - Problem 25.7
Draw the product of each...Ch. 25 - Prob. 25.8PCh. 25 - Prob. 25.9PCh. 25 - Prob. 25.10P
Ch. 25 - Prob. 25.11PCh. 25 - Prob. 25.12PCh. 25 - Prob. 25.13PCh. 25 - Prob. 25.14PCh. 25 - Prob. 25.15PCh. 25 - Prob. 25.16PCh. 25 - Prob. 25.17PCh. 25 - Problem 25.18
Write out steps to show how each of...Ch. 25 - Prob. 25.19PCh. 25 - Prob. 25.20PCh. 25 - Prob. 25.21PCh. 25 - Problem 25.22
Which nitrogen atom in each compound...Ch. 25 - Prob. 25.23PCh. 25 - Prob. 25.24PCh. 25 - Prob. 25.25PCh. 25 - Prob. 25.26PCh. 25 - Prob. 25.27PCh. 25 - Problem 25.28
Draw the major product formed in...Ch. 25 - Prob. 25.29PCh. 25 - Prob. 25.30PCh. 25 - Problem 25.31
Devise a synthesis of each compound...Ch. 25 - Prob. 25.32PCh. 25 - Problem 25.33
What starting materials are needed...Ch. 25 - Problem 25.34
(a) What two components are needed...Ch. 25 - Prob. 25.35PCh. 25 - Prob. 25.36PCh. 25 - 25.37 Varenicline (trade name Chantix) is a drug...Ch. 25 - Give a systematic or common name for each...Ch. 25 - Prob. 25.39PCh. 25 - 25.40 How many stereogenic centers are present in...Ch. 25 - 25.41 Rank the compounds in each group in order of...Ch. 25 - 25.42 Decide which atom in each molecule is most...Ch. 25 - 25.43 Explain why pyrimidine is less basic than...Ch. 25 - 25.44 Rank the nitrogen atoms in each compound in...Ch. 25 - 25.45 Explain why nitroaniline is a stronger base...Ch. 25 - 25.46 Explain the observed difference in the...Ch. 25 - 25.47 Why is pyrrole more acidic than...Ch. 25 - Prob. 25.48PCh. 25 - Prob. 25.49PCh. 25 - Prob. 25.50PCh. 25 - 25.51 How would you separate toluene , benzoic...Ch. 25 - 25.52 Draw the products formed when methylaniline ...Ch. 25 - Prob. 25.53PCh. 25 - Prob. 25.54PCh. 25 - 25.55 Draw the organic products formed in each...Ch. 25 - Prob. 25.56PCh. 25 - 25.57 Identify A, B, and C, three intermediates in...Ch. 25 - Prob. 25.58PCh. 25 - Prob. 25.59PCh. 25 - 25.60 A chiral amine A having the configuration...Ch. 25 - 25.61 Draw a stepwise mechanism for each...Ch. 25 - 25.62 Draw a stepwise mechanism for the following...Ch. 25 - Prob. 25.63PCh. 25 - 25.64 Tertiary aromatic amines react with and ...Ch. 25 - 25.65 Devise a synthesis of each compound from...Ch. 25 - Prob. 25.66PCh. 25 - Prob. 25.67PCh. 25 - Prob. 25.68PCh. 25 - Prob. 25.69PCh. 25 - 25.70 Devise a synthesis of each biologically...Ch. 25 - 25.71 Devise a synthesis of each compound from...Ch. 25 - 25.72 Three isomeric compounds A, B, and C, all...Ch. 25 - 25.73 Treatment of compound D with LiAlH4 followed...Ch. 25 - Prob. 25.74PCh. 25 - 25.75 Rank the following compounds in order of...Ch. 25 - Prob. 25.76PCh. 25 - Prob. 25.77PCh. 25 - Prob. 25.78P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry.[4 only] CH3 A. B. HNO H₂Pt H₂SO4 hano NaN 1. LIAH ether Br 4 2 H₂O C. D. E. CH3CH2-CH2CH3 + HCl Br NH₂ CH3 ON CH-CH3 Br HNOZ CUCI 11,504 HC) 1. HNO H SO NH₂ 2 UMarrow_forwardConsider the Grignard reaction below to answer the following questions. A Mgar 1. ether + MyC CH3 2H3O C B a. The electrophile in this reaction is: b. The nucleophile in this reaction is: c. The alcohol product can be classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol HO CH3 CHarrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry A. CH₂OH PCC CH2Cl2 HOO B. H KCN HCN of b C. 1. CH,MgBr, ether 2 HO* D. Choose the BEST reagent for carrying out each of the following conversions. CO₂CH3 CO₂CH3 OH CO₂H сон ن نے a. LiAlH4, ether at abinayo iss c b. NaBH4, ethanol C. CrO3, pyridine d. H₂/Pd d notsiolarrow_forward
- Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forwardWhat is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forwardIn the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forward
- QUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forwardBy malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forward
- oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forwardWhat type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License