
Concept explainers
(a)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and
Answer to Problem 25.43P
The structure that corresponds to the given name is,
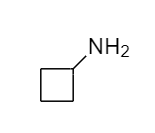
Explanation of Solution
The name of the given molecule suggests that a –NH2 is bonded to cyclobutane ring and cyclobutane consists of four carbon atoms. Therefore, the structure of the given compound is,

The structure that corresponds to the given name is shown.
(b)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
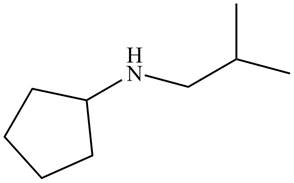
Figure 1
Explanation of Solution
The name of the given molecule suggests that a nitrogen atom is bonded to cyclopentane and isobutyl group. Cyclopentane consists of five carbon atoms. Therefore, the structure of the given compound is,
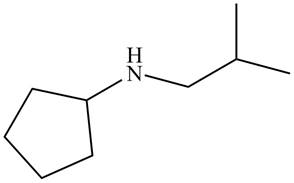
Figure 1
The structure that corresponds to the given name is shown in Figure 1.
(c)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
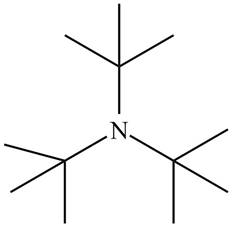
Figure 2
Explanation of Solution
The name of the given compound is tri-tert-butylamine. Prefix ‘tri’ shows that a nitrogen atom is bonded to three tert-butyl groups. Therefore, the structure of the given compound is,

Figure 2
The structure that corresponds to the given name is shown in Figure 2.
(d)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
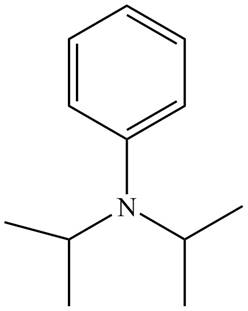
Figure 3
Explanation of Solution
The name of the given compound is N, N-diisopropylaniline. Prefix ‘di’ shows that a nitrogen atom is bonded to two isopropyl groups and it is bonded to six membered

Figure 3
The structure that corresponds to the given name is shown in Figure 3.
(e)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
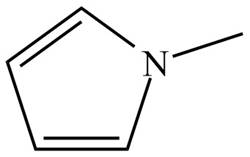
Figure 4
Explanation of Solution
The name of the given compound is N-methylpyrrole. Pyrrole is the parent chain of the given compound. It is an aromatic five membered ring which contains nitrogen atom and two double bonds. The nitrogen atom is bonded to methyl group. Therefore, the structure of the given compound is,
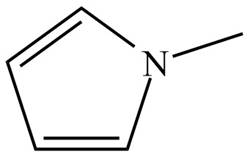
Figure 4
The structure that corresponds to the given name is shown in Figure 4.
(f)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
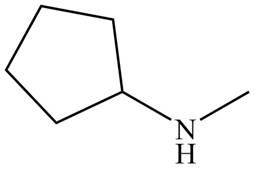
Figure 5
Explanation of Solution
The name of the given compound is N-methylcyclopentanamine. The name suggests that nitrogen atom is bonded to cyclopentane and methyl group. Therefore, the structure of the given compound is,

Figure 5
The structure that corresponds to the given name is shown in Figure 5.
(g)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
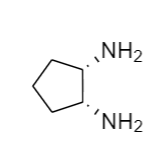
Explanation of Solution
The name of the given molecule suggests that a two–NH2 is bonded to cyclopentane ring in a cis position and cyclobutane consists of five carbon atoms. Therefore, the structure of the given compound is,

The structure that corresponds to the given name is shown.
(h)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
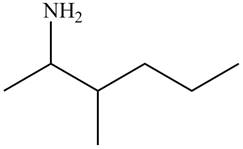
Figure 6
Explanation of Solution
The name of the given compound is
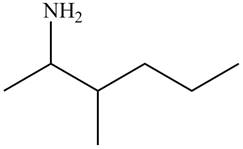
Figure 6
The structure that corresponds to the given name is shown in Figure 6.
(i)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
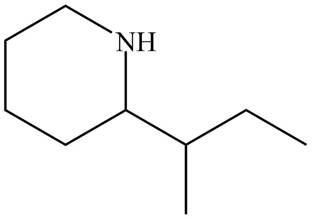
Figure 7
Explanation of Solution
The name of the given compound is
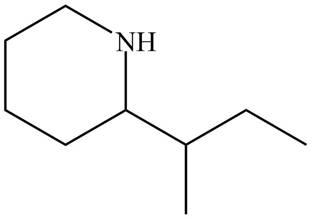
Figure 7
The structure that corresponds to the given name is shown in Figure 7.
(j)
Interpretation: The structure that corresponds to the given name is to be drawn.
Concept introduction: One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is addition of substituents at appropriate carbon atoms.
Answer to Problem 25.43P
The structure that corresponds to the given name is,
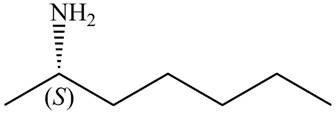
Figure 8
Explanation of Solution
The name of the given compound is (S)-heptan-

Figure 8
The structure that corresponds to the given name is shown in Figure 8.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry-Package(Custom)
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





