
(a)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction:
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
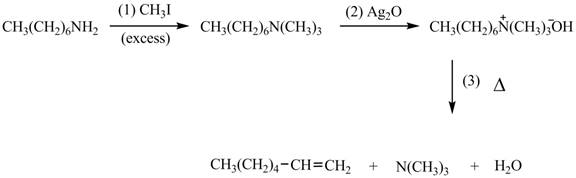
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
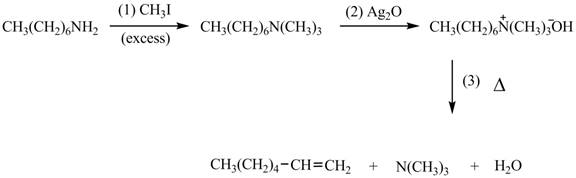
Figure 1
The products formed by the reaction of given compound with excess
(b)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
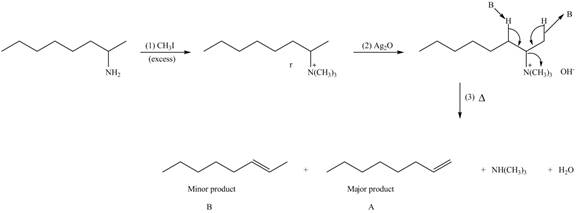
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
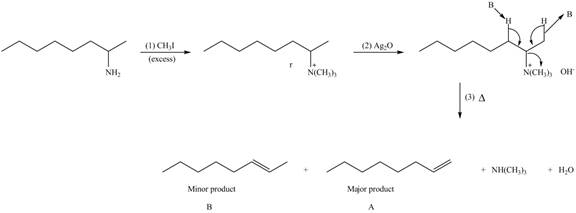
Figure 2
The products formed by the reaction of given compound with excess
(c)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
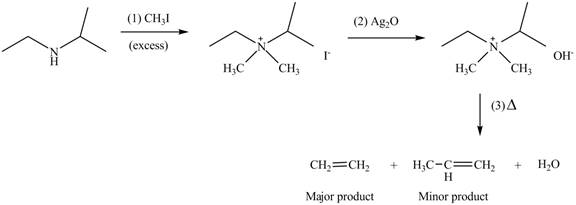
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess

Figure 3
The products formed by the reaction of given compound with excess
(d)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
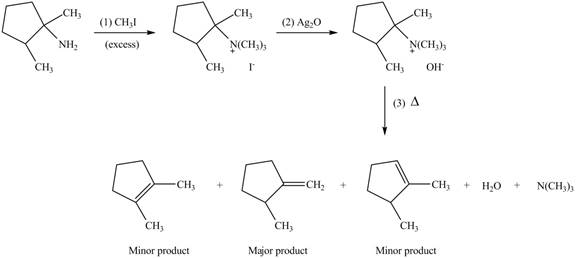
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess

Figure 4
The products formed by the reaction of given compound with excess
(e)
Interpretation: The products formed by the reaction of given compound with excess
Concept introduction: Amines on reaction with excess
Answer to Problem 25.62P
The products formed by the reaction of given compound with excess
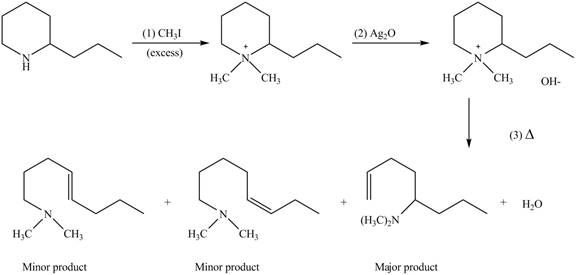
Explanation of Solution
Amines on reaction with excess
The products formed by the reaction of given compound with excess
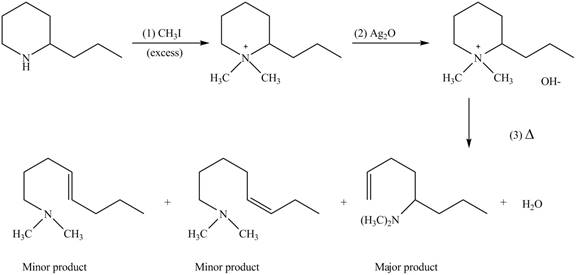
Figure 5
The products formed by the reaction of given compound with excess
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry-Package(Custom)
- drawing, no aiarrow_forwardDraw the major organic product when each of the bellow reagents is added to 3,3-dimethylbutere. ✓ 3rd attempt Part 1 (0.3 point) H.C CH CH + 1. BHG THF 210 NaOH NJ 10000 Part 2 (0.3 point) HC- CH HC 2741 OH a Search 1. He|DA HO 2. NIBH さ 士 Ju See Periodic Table See Hint j = uz C H F F boxarrow_forwardSynthesis of 2-metilbenzimidazol from 1,2-diaminobenceno y propanona.arrow_forward
- Predict the product of the following reaction. 1st attempt HI 1 product 50300 Jul See Periodic Table See Hint P Br 石尚 Iarrow_forwardIndicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forward
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardSynthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forward
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardIdentify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forward
