
Concept explainers
(a)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(a)
Explanation of Solution
The D-ribose is an aldopentose molecule. The carbonyl group present in D-ribose is
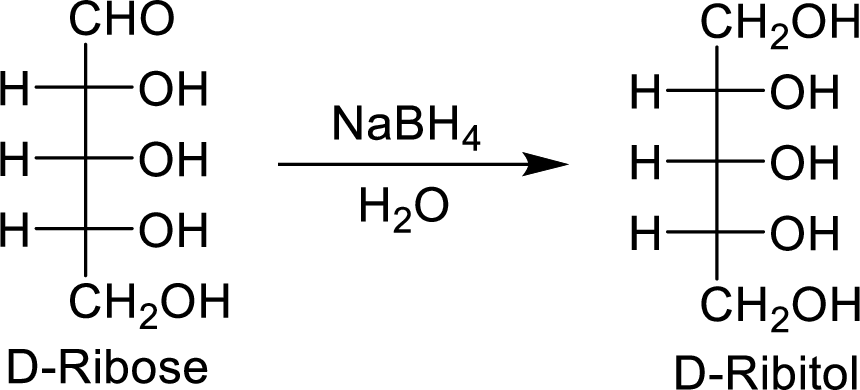
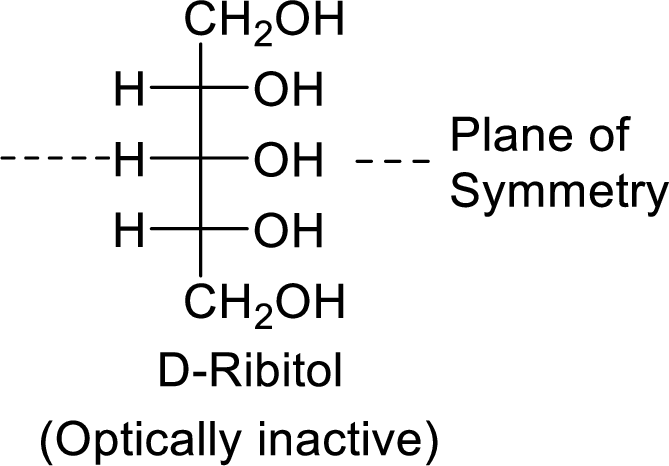
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(b)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reagent
(b)
Explanation of Solution
When the D-ribose molecule is made to react with
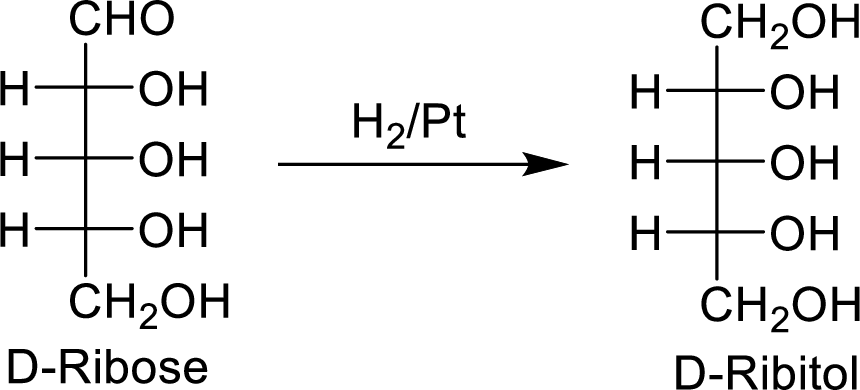
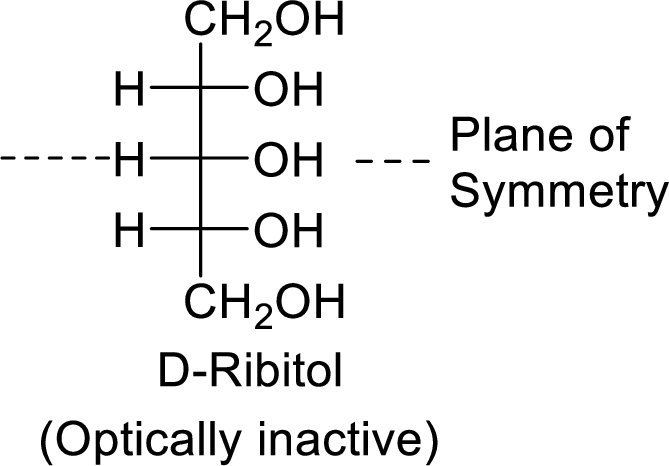
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(c)
Interpretation:
The product formed by the reaction of D-ribose with warm
Concept Introduction:
The reaction of warm
(c)
Explanation of Solution
The nitric acid (
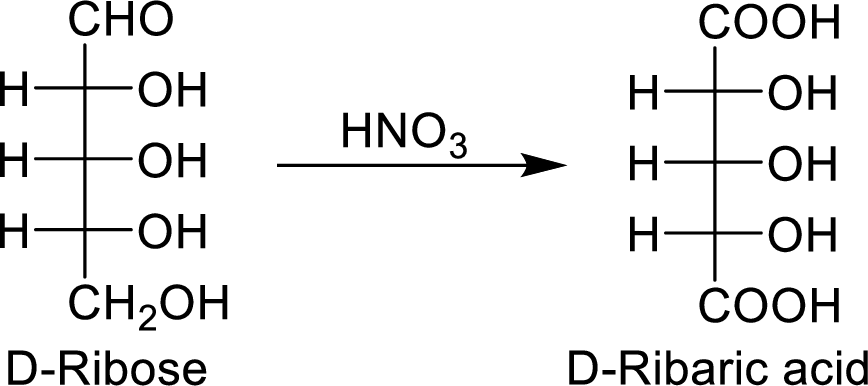
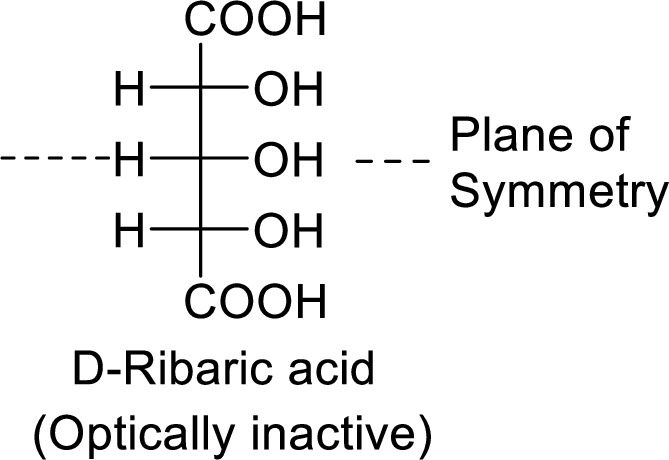
The product formed is a meso compound and shows plane of symmetry. So, it is optically inactive.
(d)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(d)
Explanation of Solution
When the D-ribose reacts with
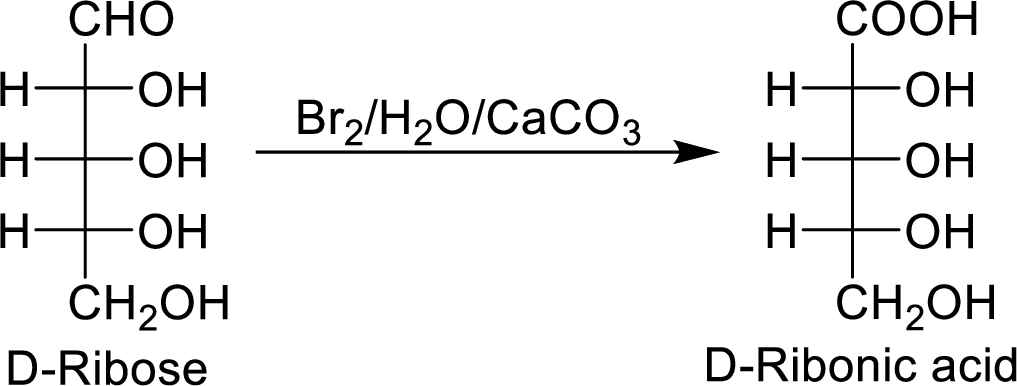
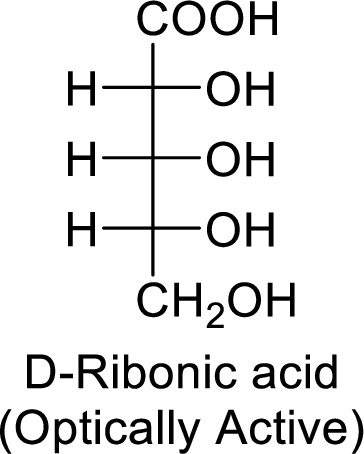
The D-ribonic acid is a chiral molecule and is optically active.
(e)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(e)
Explanation of Solution
The D-ribose molecule consumes
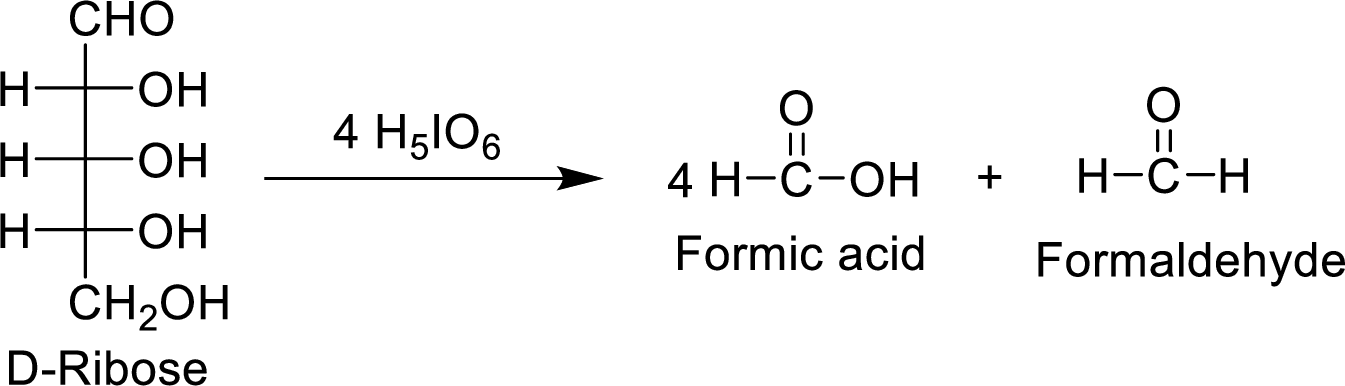
The products formed are achiral and are optically inactive.
(f)
Interpretation:
The product formed by the reaction of D-ribose with aniline (
(f)
Explanation of Solution
The D-galactose molecule reacts with aniline molecule and the aldehyde group of carbohydrate is reacted with the
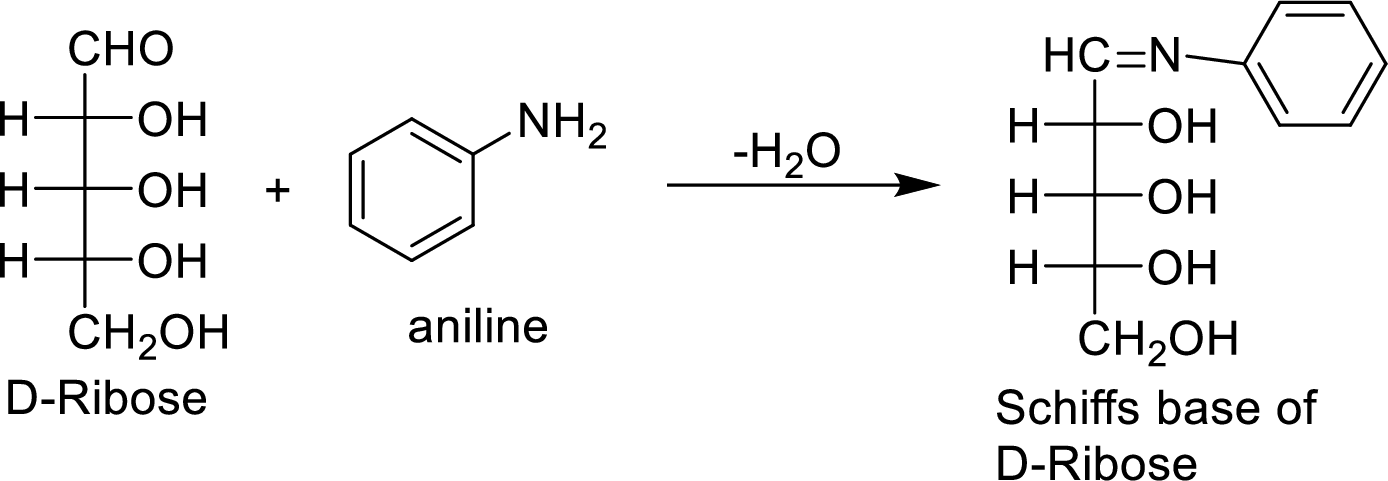
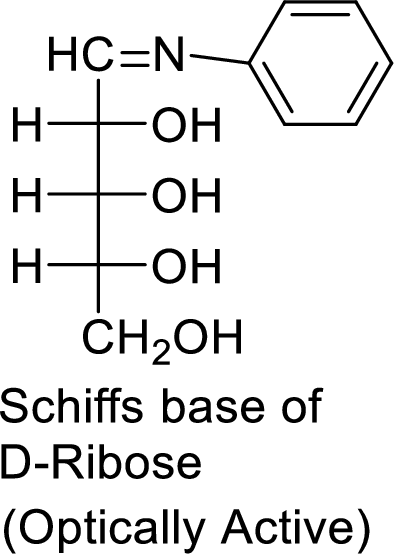
The product formed is chiral and optically active.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry, Loose-leaf Version
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

