
Concept explainers
Visible-Light Curing in Dentistry
An essential part of modern dentistry is visible-light curing (VLC), a procedure that hardens the restorative materials used in fillings, veneers, and other applications. These “curing lights" work by activating molecules known as photoinitiators within the restorative materials The photoinitiators, in turn, start a process of
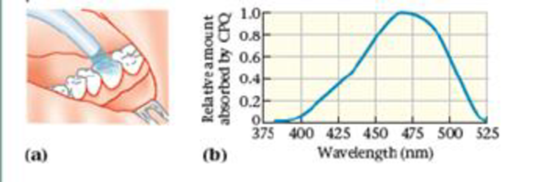
Figure 25-50
An intense beam of light cures or hardens, the restorative material used to fill a cavity. (Problems 109, 110, 111, and 112)
The most common photo initiator is camphoroquinone (CPQ). To cure CPQ in the least time, one should illuminate it with
More recently, VLC units have begun to use LEDs as their light source These lights stay cool, emit nearly all of their energy output as visible light at the desired wavelength, and provide light with an intensity as high as 1000 m W/cm2, which is about 10 times the intensity of sunlight on the surface of the Earth.
112 • Assuming the light from the VLC unit has a beam 0 50 cm in diameter, how much energy does the light deliver in 20 seconds?
- A. 0.025 J
- B. B 3 9 J
- C. C 63 J
- D. D 5000 J
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
Physics (5th Edition)
Additional Science Textbook Solutions
College Physics
Conceptual Physics (12th Edition)
Life in the Universe (4th Edition)
University Physics with Modern Physics (14th Edition)
University Physics (14th Edition)
The Cosmic Perspective Fundamentals (2nd Edition)
- Laser vision correction often uses an excimer laser that produces 193-nm electromagnetic radiation. This wavelength is extremer strongly absorbed by the cornea and athletes it in a manner that reshapes the cornea to correct vision detects. Explain how the strong absorption helps concentrate the energy in a thin layer and thus give greater accuracy in shaping the cornea. Also explain how this strong absorption limits damage to the lens and retina of the eye.arrow_forward(a) What is me ratio of the speed of red light to violet light in diamond, based on Table 25.2? (b) What is this ratio in polystyrene? (c) Which is more dispersive?arrow_forwardConstruct Your Own Problem Consider a space sail such as mentioned in Example 29.5 Construct a problem in which you calculate the light pressure on the sail in N/m2 produced by reflecting sunlight. Also calculate the force that could be produced and how much effect that would have on a spacecraft. Among the things to be considered are the intensity of sunlight, its average wavelength, the number of photons per square meter this implies, the area of the space sail, and the mass of the system being accelerated.arrow_forward
- Integrated Concepts (a) During laser vision correction, at brief burst at 193 nm ultraviolet light is projected onto the cornea of the patient. It makes a spot 1.00 mm in diameter and deposits 0.500 mJ of energy. Calculate the depth of the layer ablated, assuming the corneal tissue has the same properties as water and is initially at 34.0°C. The tissue’s temperature is increased to 100°C and evaporated without further temperature increase. (b) Does your answer imply that the shape of the cornea can be ?nely controlled?arrow_forwardWhich of the following colors of light has the highest amount of energy? A. Violet, wavelength 400 nm B. Red, wavelength 700 nm C. Yellow, wavelength 590 nm D. Green, wavelength 530 nmarrow_forward1- Why is it important to align the mirrors in a laser cavity? Explain how a gas laser cavity can be aligned using a low-powered HeNe laser.arrow_forward
- During a brown-out, the electric power company drops the voltage of the power line and the incandescent light bulbs in your house become dimmer. When this happens, the filaments in the bulbs operate at a lower than normal temperature, so a. their color is somewhat bluer than normal. b. they emit ultraviolet light. c. their color is the same as normal, but they are less bright. d. their color is somewhat redder than normal. e. they burn out more quickly.arrow_forwardI Review I Constants I Periodic Table X rays with a wavelength of 0.19 nm undergo first- order diffraction from a crystal at a 57 ° angle of incidence. Part A At what angle does first-order diffraction occur for x rays with a wavelen of 0.12 nm ? Express your answer using two significant figures. ΑΣφ ? = Submit Request Answerarrow_forwardI Review I Constants I Periodic Table X rays with a wavelength of 0.12 nm undergo first- order diffraction from a crystal at a 61 ° angle of incidence. Part A What is the angle of second-order diffraction? Express your answer using two significant figures. ? 02 = Submit Request Answerarrow_forward
- 4arrow_forwardThis question is not part of an exam it is a normal homework question Investigators measure the size of fog droplets using the diffraction of light. A camera records the diffraction pattern on a screen as the droplets pass in front of a laser, and a measurement of the size of the central maximum gives the droplet size. In one test, a 690 nmnm laser creates a pattern on a screen 30 cmcm from the droplets. If the central maximum of the pattern is 0.30 cmcm in diameter, how large is the droplet? Express your answer with the appropriate units.arrow_forwardis very effective in------------- 2 heating cancerous tumors, because tumors typically have high-water * content a. Microwave energy O b.R O cuv O d. X-ray Oarrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





