
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 25, Problem 68PCE
Predict Explain Consider the two polarization experiments shown in Figure 25-42 (a) If the incident light is unpolarized, is the transmitted intensity in case A greater than less man or the same as the transmitted intensity in case D? (b) Choose the best explanation from among the following:
- I. The transmitted intensity is the same in either case the first polarizer lets through one-half the incident intensity, and the second polarizer is at an angle θ relative to the first.
- II. Case A has a smaller transmitted intensity than case B because the first polarizer is at an angle θ relative to the incident beam.
- III. Case B has a smaller transmitted intensity than case A because the direction of polarization is rotated by an angle θ in the clockwise direction in case B.
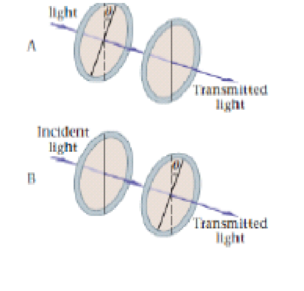
Figure 25-42
Problems 68 and 69
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2
3
Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.
Chapter 25 Solutions
Physics (5th Edition)
Ch. 25.1 - Enhance Your Understanding (Answers given at the...Ch. 25.2 - A distant galaxy is moving away from the Earth....Ch. 25.3 - If the frequency of an electromagnetic wave is...Ch. 25.4 - Prob. 4EYUCh. 25.5 - In the system shown in Figure 25-36, a vertically...Ch. 25 - Explain why the invisible man would be unable to...Ch. 25 - While wearing your Polaroid sunglasses at the...Ch. 25 - You want to check the tine while wearing your...Ch. 25 - BIO Polarization and the Ground Spider The ground...Ch. 25 - You are given a sheet of Polaroid material....
Ch. 25 - Can sound waves be polarized? Explain.Ch. 25 - At a garage sale you find a pair of Polaroid...Ch. 25 - If the electric field in an electromagnetic wave...Ch. 25 - Prob. 2PCECh. 25 - Prob. 3PCECh. 25 - Prob. 4PCECh. 25 - Give the direction (N, S, E, W, up, or down) of...Ch. 25 - Prob. 6PCECh. 25 - Prob. 7PCECh. 25 - The light year (ly) is a unit of distance commonly...Ch. 25 - Alpha Centauri, the closest star to the Sun, is...Ch. 25 - Prob. 10PCECh. 25 - A fighter jet is traveling at 515 m/s directly...Ch. 25 - A distant star is traveling directly away from...Ch. 25 - Prob. 13PCECh. 25 - Prob. 14PCECh. 25 - Prob. 15PCECh. 25 - Prob. 16PCECh. 25 - Communicating with the Voyager Spacecraft The...Ch. 25 - A father and his daughter are interested in the...Ch. 25 - Prob. 19PCECh. 25 - Prob. 20PCECh. 25 - Prob. 21PCECh. 25 - Baseball scouts often use a radar gun to measure...Ch. 25 - A state highway patrol car radar unit uses a...Ch. 25 - Prob. 24PCECh. 25 - Prob. 25PCECh. 25 - BIO Dental X-rays The X-rays produced in the...Ch. 25 - Find the frequency of green light with a...Ch. 25 - Prob. 28PCECh. 25 - How many led wavelengths ( = 705 nm) tall are you?Ch. 25 - A cell phone transmits at a frequency of 1.94 ...Ch. 25 - Microwave Oven If a microwave oven produces...Ch. 25 - BIO Human Radiation Under normal conditions,...Ch. 25 - BIO UV Radiation Ultraviolet light is typically...Ch. 25 - Prob. 34PCECh. 25 - Predict/Calculate When an electromagnetic wave...Ch. 25 - Predict/ Calculate (a) Which color of light has...Ch. 25 - Prob. 37PCECh. 25 - A television is tuned to a station broadcasting at...Ch. 25 - An AM radio stations antenna is constructed to be...Ch. 25 - Prob. 40PCECh. 25 - Find the difference in wavelength (1 2) for each...Ch. 25 - Synchrotron Frequency In one portion of a...Ch. 25 - Prob. 43PCECh. 25 - Prob. 44PCECh. 25 - Prob. 45PCECh. 25 - What is the rms value of the electric field in a...Ch. 25 - The magnetic field in an electromagnetic wave has...Ch. 25 - What is the maximum value of the electric field in...Ch. 25 - What is the maximum value of the electric field in...Ch. 25 - Predict/Calculate Electromagnetic wave 1 has a...Ch. 25 - A 75-kW radio station broadcasts its signal...Ch. 25 - At what distance will a 45-W lightbulb have the...Ch. 25 - What is the ratio of the sunlight intensity...Ch. 25 - Predict/Calculate In the following, assume that...Ch. 25 - Prob. 55PCECh. 25 - Prob. 56PCECh. 25 - Sunlight Intensity After filtering through the...Ch. 25 - Predict/Calculate (a) Find the electric and...Ch. 25 - Prob. 59PCECh. 25 - BIO You are standing 2.5 m from a 150-W lightbulb....Ch. 25 - Prob. 61PCECh. 25 - Find the rms electric and magnetic fields al a...Ch. 25 - Prob. 63PCECh. 25 - Prob. 64PCECh. 25 - Prob. 65PCECh. 25 - BIO Laser Surgery Each pulse produced by an...Ch. 25 - Prob. 67PCECh. 25 - Predict Explain Consider the two polarization...Ch. 25 - Predict/Explain Consider the two polarization...Ch. 25 - An incident beam of light with an intensityl0....Ch. 25 - Vertically polarized light with an intensity of...Ch. 25 - A person riding in a boat observes that the...Ch. 25 - Unpolarized light passes through two polarizers...Ch. 25 - In Problem 73, what should be the angle between...Ch. 25 - Unpolarized light is incident with intensity /0 on...Ch. 25 - Predict/Calculate A beam of vertically polarized...Ch. 25 - Predict/Calculate Repeat Problem 76, this time...Ch. 25 - BIO Predict/Calculate Optical Activity Optically...Ch. 25 - A helium-noon laser omits a beam of unpolarizod...Ch. 25 - Referring to Figure 25-46, suppose that filter 3...Ch. 25 - Prob. 81GPCh. 25 - CE If sailors of the future use radiation pressure...Ch. 25 - Prob. 83GPCh. 25 - BIO Radiofrequency Ablation In radiofrequency (RF)...Ch. 25 - Predict/Calculate At a particular instant of time,...Ch. 25 - Predict/Calculate A light beam traveling in the...Ch. 25 - Figure 25-47 shows four polarization experiments...Ch. 25 - Lightning and Thunder During a thunderstorm a bolt...Ch. 25 - Prob. 89GPCh. 25 - Prob. 90GPCh. 25 - Predict/Calculate Suppose the distance to the...Ch. 25 - BIO Predict/Calculate Consider the physical...Ch. 25 - BIO Polaroid Vision in a Spider Experiments show...Ch. 25 - A state highway patrol car radar unit uses a...Ch. 25 - What is the ratio of the sunlight intensity...Ch. 25 - What area is needed for a solar collector to...Ch. 25 - Prob. 97GPCh. 25 - Three polarizers are arranged as shown in Figure...Ch. 25 - Prob. 99GPCh. 25 - Orbital Drift The radiation pressure exerted by...Ch. 25 - A lightbulb emits light uniformly in all...Ch. 25 - Radio Reception A 125-kW radio station broadcasts...Ch. 25 - Light Rocket Stranded 12 m from your spacecraft,...Ch. 25 - A typical home may require a total of 2.00 103...Ch. 25 - Prob. 105GPCh. 25 - Predict/Calculate A typical laser used in...Ch. 25 - Four polarizers are set up so that the...Ch. 25 - BIO Optical Activity of Sugar The sugar...Ch. 25 - Visible-Light Curing in Dentistry An essential...Ch. 25 - Visible-Light Curing in Dentistry An essential...Ch. 25 - Visible-Light Curing in Dentistry An essential...Ch. 25 - Visible-Light Curing in Dentistry An essential...Ch. 25 - Predict/Calculate Referring to Example 25-12...Ch. 25 - Referring to Example 25-12 Suppose the incident...
Additional Science Textbook Solutions
Find more solutions based on key concepts
26. Compute 3.24 m + 0.532 m to the correct number of significant figures.
3.7 m
3.77 m
3.772 m
3.7720 m
College Physics: A Strategic Approach (3rd Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forwardpls help on thesearrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forward
- pls help on allarrow_forwardpls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Polarization of Light: circularly polarized, linearly polarized, unpolarized light.; Author: Physics Videos by Eugene Khutoryansky;https://www.youtube.com/watch?v=8YkfEft4p-w;License: Standard YouTube License, CC-BY