
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337670418
Author: Kotz
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Chapter 24.5, Problem 1.3ACP
Interpretation Introduction
Interpretation:
The molecular geometry around the phosphorous atom in a phosphorothioate group has to be identified.
Concept introduction: In phosphorothioate group phosphrous atom bonded with surrounding atoms oxygen and sulphur as shown below.
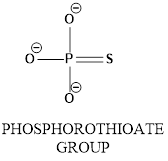
In the diagram shows
Tetrahedral geometry: A tetrahedral has a central atom which is surrounded by four other atoms. In tetrahedral geometry the bond angle is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O
What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete?
Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water?
Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?
What mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07
What is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/mol
Chapter 24 Solutions
Chemistry & Chemical Reactivity
Ch. 24.1 - Draw the Lewis structure for the tripeptide...Ch. 24.3 - What is the sequence of the strand of DNA...Ch. 24.3 - Prob. 24.3CYUCh. 24.5 - Kynamro has the hydrogen bonding sequence:...Ch. 24.5 - The formula of Kynamro is...Ch. 24.5 - Prob. 1.3ACPCh. 24.5 - Prob. 2.1ACPCh. 24.5 - Prob. 2.2ACPCh. 24.5 - Prob. 2.3ACPCh. 24 - (a) Draw the Lewis structure for the amino acid...
Ch. 24 - (a) Draw the Lewis structure for the amino acid...Ch. 24 - Prob. 3PSCh. 24 - Prob. 4PSCh. 24 - Draw Lewis structures for the two dipeptides that...Ch. 24 - Do the amino acid sequences: valine-asparagine and...Ch. 24 - Draw the Lewis structure for the tripeptide...Ch. 24 - Prob. 8PSCh. 24 - Prob. 9PSCh. 24 - Prob. 10PSCh. 24 - Prob. 11PSCh. 24 - Prob. 12PSCh. 24 - (a) Draw the structural formula for the sugar...Ch. 24 - (a) Draw the structural formula for the sugar -D-2...Ch. 24 - Prob. 15PSCh. 24 - Prob. 16PSCh. 24 - Given the following nucleotide sequence in DNA:...Ch. 24 - Given the following nucleotide sequence in DNA: 5'...Ch. 24 - Prob. 19PSCh. 24 - If a drop of oleic acid is added to a dish of...Ch. 24 - What structure do all steroids have in common?Ch. 24 - Prob. 22PSCh. 24 - Prob. 23PSCh. 24 - The chemical equation for the fermentation of...Ch. 24 - Prob. 25PSCh. 24 - Prob. 26PSCh. 24 - Prob. 27GQCh. 24 - Prob. 28GQCh. 24 - Prob. 29GQCh. 24 - Prob. 30GQCh. 24 - Prob. 31GQCh. 24 - There are 41 = 4 mononucleotides of DNA, there are...Ch. 24 - Prob. 33GQCh. 24 - The first step of the metabolic process known as...Ch. 24 - Prob. 35ILCh. 24 - Insulin is a protein important in the metabolism...Ch. 24 - Prob. 37SCQCh. 24 - Prob. 38SCQCh. 24 - Do the DNA sequences ATGC and CGTA represent the...Ch. 24 - Prob. 41SCQCh. 24 - Which of the following statements is/are true? (a)...
Knowledge Booster
Similar questions
- The rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?arrow_forwardHandwritten pleasearrow_forwardChoose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forward
- The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forwardChoose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forwardPlease answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forwardHow should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve. Software: Excel Spreadsheets Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ugarrow_forwardProvide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly, but do not use italics in Canvas.arrow_forward
- The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forwardFor reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning