
Concept explainers
Interpretation:
The amount of ATP which is generated per gram of glucose in comparison to the amount of ATP generated per gram of arachidic acid during catabolism should be determined along with whether the result supports the fact that lipids are more effective energy-storing molecules in comparison to carbohydrates.
Concept introduction:
Carbohydrates are considered to be less effective than lipids in terms of releasing per gram consumption. But still, food rich in carbohydrate contents are more preferred over fats containing food. Energy produced by food containing high content of fat is almost double the amount of energy produced by food containing high content of carbohydrates.
Answer to Problem 99CP
The amount of which is generated per gram of glucose = 0.18mole ATP.
The amount of ATP generated per gram of arachidic acid during catabolism is equal to 0.43 mole ATP.
The number of ATP produced by complete catabolism of fat (fatty acid) is much higher than that of glucose (carbohydrate). Also, the energy produced by fat (fatty acid) is almost double the amount of energy produced by glucose (carbohydrate) by the complete catabolism. These results support the fact that lipids are more effective energy-storing molecules in comparison to carbohydrates.
Explanation of Solution
Glucose (a carbohydrate) starts with the glycolysis pathway which converts glucose to pyruvate.The initiation of the glycolysis process requires energy in the form of ATP. Total
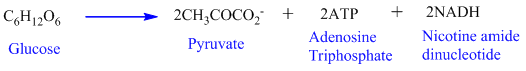
Transition reaction: On oxidation, pyruvate converted to acetyl CoA.
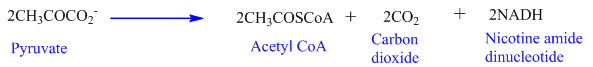
Citric acid Cycle:

From all the three reactions, total
Thus, the total
To calculate the amount of
As, fats (lipids) are metabolized within the body through
The first step is the investment of energy when
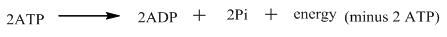
The number of acetyl
Each molecule
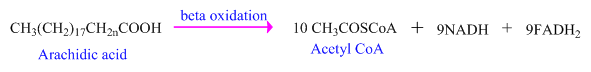
Acetyl
Every Acetyl
Thus, the total number of ATP generated during the cycle is
To calculate the amount of
From the calculation, it is clear that the number of ATP produced by complete catabolism of fat (fatty acid) is much higher than that of glucose (carbohydrate). Also, the energy produced by fat (fatty acid) is almost double the amount of energy produced by glucose (carbohydrate) by the complete catabolism. And these results supportthe factthat lipids (fats) store energy more effectively than carbohydrates.
Want to see more full solutions like this?
Chapter 24 Solutions
General, Organic, and Biological Chemistry - 4th edition
- What is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forwardIdentify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward4:45 PM Tue Apr 1 K 77% Problem 9 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. :0: H Select to Add Arrows HI CH3OH H+ ·HO CH3OH, H+ 0:0 H H Select to Add Arrows tion Versirate CH3OH, H* Select to Draw Productarrow_forward
- Can I please get help with this graph? If you can show exactly where it needs to pass through.arrow_forwardG 1. PPh3, THF 2. 3. LiH, THF ' THF H Harrow_forwardPlease EnCircle or Fill-In your Choice CLEARLY: 21. Please Sketch the intermediates for each step below. Draw the Product which would result from the following series of reactions. Name each Type of Rx: 1. Br2, FeBr3 2. Mg, ether 3. ethylene oxide 4. H₂O+ 5. PBr3 6. Mg, ether 7. 8. H3O+, heat (-H₂O 9. HF ?arrow_forward
- Can I please get help with this question. All required information should be in data table.arrow_forwardesc For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. Major Product: Explanation Check C ☐ + X NaOH Br F1 F2 80 F3 F4 F5 F6 1 ! @ 2 3 $ 4 % 5 Q W LU E S D A F7 * C Click and dr drawing a 2025 McGraw Hill LLC. All Rights Reserv ►II F8 4 F9 6 7 8 9 R T Y U LL F G H Jarrow_forwardCalculate equilibrium concentrations for the following reaction:N2 (g) + O2 (g) ⇋ 2 NO (g) Kc = 0.10 at 2273K initially [N2] = 0.200M; [O2] = 0.200arrow_forward
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardThe following reaction is run in which the initial conditions include only methane (CH4) at a concentration of0.115 M. Once equilibrium was established, the concentration of acetylene (C2H2) was measured to be 0.035M. What is the value of the equilibrium constant, K?2 CH4 (g) ⇋ C2H2 (g) + 3 H2 (g)arrow_forwardCalculate the equilibrium concentration of carbon dioxide for the following reaction:2 COF2 (g) ⇋ CF4 (g) + CO2 (g) Kc = 2.00 at 10.00 °C. at equilibrium [COF2] = 0.255M; [CF4] = 0.118Marrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





