
(a)
Interpretation:
Hydrogen bonding between 2 water molecules should be depicted.
Concept introduction:
All types of molecules have some kind of attractive force that keeps them together. Polar molecules that have elements that are high in electro negativity like nitrogen, oxygen and fluorine directly attached to hydrogen atom, exhibit hydrogen bonding. They are held together by hydrogen bonding between molecules.
Hydrogen bonding is a special kind of dipole-dipole bond.
(a)
Answer to Problem 98A
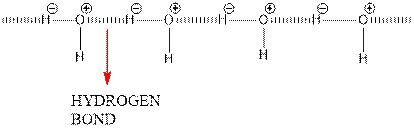
Hydrogen bonding between 2 water molecules is shown below.
Explanation of Solution
In case of
Since oxygen is electronegative centre, it can easily attract hydrogen molecule of other water molecule and hence a partial positive charge develops on it and a partial negative charge develops on hydrogen. This results in intermolecular hydrogen bonding.
(b)
Interpretation:
Hydrogen bonding between 2 ammonia molecules should be depicted.
Concept introduction:
All types of molecules have some kind of attractive force that keeps them together. Polar molecules that have elements that are high in electro negativity like nitrogen, oxygen and fluorine directly attached to hydrogen atom, exhibit hydrogen bonding. They are held together by hydrogen bonding between molecules.
Hydrogen bonding is a special kind of dipole-dipole bond.
(b)
Answer to Problem 98A
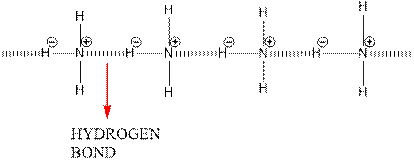
Hydrogen bonding between 2 ammonia molecules is shown below.
Explanation of Solution
In case of
Since nitrogen is electronegative centre, it can easily attract hydrogen molecule of other ammonia molecule and hence a partial positive charge develops on it and a partial negative charge develops on hydrogen. This results in intermolecular hydrogen bonding.
(c)
Interpretation:
Hydrogen bonding between water and ammonia molecules should be depicted.
Concept introduction:
All types of molecules have some kind of attractive force that keeps them together. Polar molecules that have elements that are high in electro negativity like nitrogen, oxygen and fluorine directly attached to hydrogen atom, exhibit hydrogen bonding. They are held together by hydrogen bonding between molecules.
Hydrogen bonding is a special kind of dipole-dipole bond.
(c)
Answer to Problem 98A
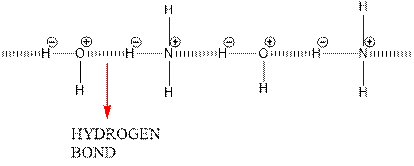
Hydrogen bonding between water and ammonia molecules is shown below.
Explanation of Solution
In case of
Similarly in case of
Since oxygen is electronegative centre, it can easily attract hydrogen molecule of other ammonia molecule and nitrogen can attract hydrogen molecules of water molecules and hence a partial positive charge develops on oxygen and nitrogen atom. A partial negative charge develops on hydrogen. This results in intermolecular hydrogen bonding.
Chapter 24 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Anatomy & Physiology (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: The Central Science (14th Edition)
Campbell Biology (11th Edition)
- Including activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward
- (Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forward
- A 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forward
- The compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





