
College Physics
2nd Edition
ISBN: 9780134601823
Author: ETKINA, Eugenia, Planinšič, G. (gorazd), Van Heuvelen, Alan
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 24, Problem 64GP
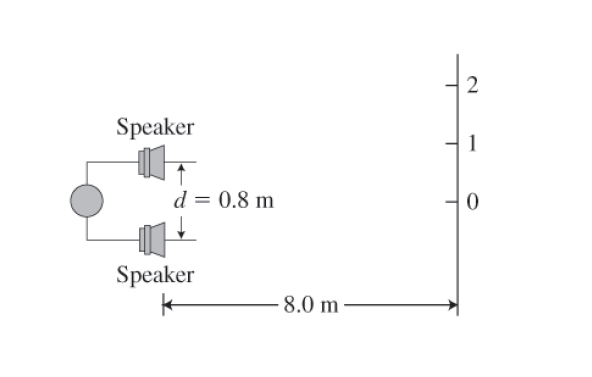
Sound from speakers Two stereo speakers separated by a distance of 0.8 m play the same musical note at frequency 1000 Hz. A listener starts from position 0 (Figure P24.64) and walks along a line parallel to the speakers (a) Can the listener easily hear the sound at position 0? Explain (b) Calculate the distance from position 0 to positions 1 and 2 where intense sound is also heard The speed of sound is 340 m/s.
Figure P24.64
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can someone help me
A particle in a box between x=0 and x=6 has the wavefunction Psi(x)=A sin(2πx). How muchenergy is required for the electron to make a transition to Psi(x)= A’ sin(7π x/3). Draw anapproximate graph for the wavefunction. Find A and A'
A proton is moving with 10^8 m/s speed. Find the De Broglie wavelength associated with theproton and the frequency of that wave.
Chapter 24 Solutions
College Physics
Ch. 24 - Review Question 24.1 Explain why we observe...Ch. 24 - Prob. 2RQCh. 24 - Review Question 24.3 How do the locations of the...Ch. 24 - Review Question 24.4 If we look through a grating...Ch. 24 - Review Question 24.5 Equation (24.6),...Ch. 24 - Review Question 24.6 Stars are so far away that...Ch. 24 - Prob. 7RQCh. 24 - Multiple Choice Questions
1. You shine a...Ch. 24 - Multiple Choice Questions When you shine a very...Ch. 24 - Prob. 3MCQ
Ch. 24 - Multiple Choice Questions If you add a third slit...Ch. 24 - Multiple Choice Questions
5. Why don’t two...Ch. 24 - Multiple Choice Questions You shine a laser beam...Ch. 24 - Multiple Choice Questions
7. What does the...Ch. 24 - Prob. 8MCQCh. 24 - Multiple Choice Questions You shine a green laser...Ch. 24 - 10. Describe a double-slit interference experiment...Ch. 24 - You are investigating a pattern produced on a...Ch. 24 - 12. Give examples of phenomena that can be...Ch. 24 - 13. Give examples of phenomena that cannot be...Ch. 24 - Prob. 14CQCh. 24 - 15. Draw a point-like source of light. What is the...Ch. 24 - Draw two coherent light sources next to each...Ch. 24 - 17. Use the wave front representation to explain...Ch. 24 - 18. Use the wave front representation to explain...Ch. 24 - Compare the interference pattern produced by two...Ch. 24 - Draw 10 coherent point-like sources of light...Ch. 24 - If you see green light of 520-nm wavelength when...Ch. 24 - 22. Imagine that you have a very thin uniform oil...Ch. 24 - (a) Draw a picture of what you will see on a...Ch. 24 - Describe three situations that you can analyze...Ch. 24 - Why can you hear a person who is around a corner...Ch. 24 - 26 Astronomers often called the resolution limit...Ch. 24 - 24.1 and 24.2 Youngs double-slit experiment and...Ch. 24 - 24.1 and 24.2 Youngs double-slit experiment and...Ch. 24 - 24.1 and 24.2 Young’s double-slit experiment and...Ch. 24 - 24.1 and 24.2 Youngs double-slit experiment and...Ch. 24 - 24.1 and 24.2 Young’s double-slit experiment and...Ch. 24 - 24.1 and 24.2 Youngs double-slit experiment and...Ch. 24 - 24.1 and 24.2 Youngs double-slit experiment and...Ch. 24 - Gratings: an application of interference Light of...Ch. 24 - 24.3 Gratings: an application of interference...Ch. 24 - 24.3 Gratings: an application of interference
12....Ch. 24 - Gratings: an application of interference Only half...Ch. 24 - 24.3 Gratings: an application of interference...Ch. 24 - 24.3 Gratings: an application of interference...Ch. 24 - 24.3 Gratings: an application of interference
18....Ch. 24 - 24.4 Thin-film interference
20. * Representing...Ch. 24 - 24.4 Thin-film interference
21. * Oil film on...Ch. 24 -
24.4 Thin-film interference
22. * Soap bubble 1 ...Ch. 24 - 24.4 Thin-film interference * Soap bubble 2 soap...Ch. 24 - 24.4 Thin-film interference
24. * Thin-film coated...Ch. 24 - Thin-film interference * Thin-film coated glass...Ch. 24 - 24.4 Thin-film interference
26. Two flat glass...Ch. 24 - 24.5 Diffraction of light * Explain diffraction...Ch. 24 - 24.5 Diffraction of light * How did we derive it?...Ch. 24 - 24.5 Diffraction of light
31. * Explain a white...Ch. 24 - 24.5 Diffraction of light Light of wavelength 630...Ch. 24 - 24.5 Diffraction of light * Light of wavelength of...Ch. 24 - 24.5 Diffraction of light * Sound diffraction...Ch. 24 - 24.5 Diffraction of light * Light of wavelength...Ch. 24 - Prob. 36PCh. 24 - 24.6 Resolving power
37. Resolution of telescope ...Ch. 24 - Resolving power * Laser light of wavelength 630 nm...Ch. 24 - Resolving power * Size of small bead Infrared...Ch. 24 - Resolving power * Resolution of telescope How will...Ch. 24 - Resolving power * Detecting visual binary stars...Ch. 24 - Prob. 42PCh. 24 - 24.6 Resolving power
43 * Draw a graphical...Ch. 24 - 24.7 Skills for applying the wave model of...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - Prob. 48PCh. 24 - Prob. 50PCh. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of...Ch. 24 - 24.7 Skills for applying the wave model of...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - 24.7 Skills for applying the wave model of light *...Ch. 24 - * Monochromatic light passes through two slits and...Ch. 24 - 64. Sound from speakers Two stereo speakers...Ch. 24 - Prob. 65GPCh. 24 - 66. Diffraction of water waves entering a harbor ...Ch. 24 - ** Variable thickness wedge A wedge of glass of...Ch. 24 - Prob. 69GPCh. 24 - Looking at Moon rocks You have a home telescope...Ch. 24 - * BIO EST Diffraction-limited resolving power of...Ch. 24 - 72. * Resolving sunspots You are looking at...Ch. 24 - s Mare Imbrium The outermost ring of mountains...Ch. 24 - * Can you see atoms with a light-based microscope?...Ch. 24 - * Detecting insects by diffraction of sound A...Ch. 24 - BIO What is 20/20 vision? Vision is often measured...Ch. 24 -
BIO What is 20/20 vision? Vision is often...Ch. 24 - BIO What is 20/20 vision? Vision is often measured...Ch. 24 - BIO What is 20/20 vision? Vision is often measured...Ch. 24 - BIO What is 20/20 vision? Vision is often measured...Ch. 24 - Thin-film window coatings for energy conservation...Ch. 24 - Thin-film window coatings for energy conservation...Ch. 24 - Thin-film window coatings for energy conservation...Ch. 24 - Thin-film window coatings for energy conservation...Ch. 24 - Thin-film window coatings for energy conservation...
Additional Science Textbook Solutions
Find more solutions based on key concepts
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Hydrogen gas can be prepared in the laboratory by a single-displacement reaction in which solid zinc reacts wit...
Introductory Chemistry (6th Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Find the wavelength of the photon if a (Li--) electron makes a transition from n=4 to n=3. Findthe Bohr radius for each state.arrow_forwardA photon with wavelength 3000 nm hits a stationary electron. After the collision electron isscattered to 60 degrees. Find the wavelength and frequency of the scattered photon.arrow_forwardA metal has threshold frequency 10^15. Calculate the maximum kinetic energy of the ejectedelectron if a laser beam with wavelength 1.5 10^-7 m is projected on the metal.arrow_forward
- Determine the direction of the vector V, B, or ♬ that is missing from the pair of vectors shown in each scenario. Here, u is the velocity vector of a moving positive charge, B is a constant and uniform magnetic field, and F is the resulting force on the moving charge. 1. 2. 3. B OB F 4. ↑F F 5. 怔 ↑ ↑F Answer Bank 6. ↑ TE Farrow_forwardTwo point charges (+9.80 nC and -9.80 nC) are located 8.00 cm apart. Let U=0 when all of the charges are separated by infinite distances. What is the potential energy if a third point charge q=-4.20 nC is placed at point b? 8.00 cm 8.00 cm 4.00 +4.00 +4.00- cm cm cm HJarrow_forward! Required information Two chloride ions and two sodium ions are in water, the "effective charge" on the chloride ions (CI¯) is −2.00 × 10-21 C and that of the sodium ions (Na+) is +2.00 x 10-21 C. (The effective charge is a way to account for the partial shielding due to nearby water molecules.) Assume that all four ions are coplanar. CT Na+ Na+ 30.0° 45.0% с сг L. where a = 0.300 nm, b = 0.710 nm, and c = 0.620 nm. What is the direction of electric force on the chloride ion in the lower right-hand corner in the diagram? Enter the angle in degrees where positive indicates above the negative x-axis and negative indicates below the positive x-axis.arrow_forward
- A pendulum has a 0.4-m-long cord and is given a tangential velocity of 0.2 m/s toward the vertical from a position 0 = 0.3 rad. Part A Determine the equation which describes the angular motion. Express your answer in terms of the variable t. Express coefficients in radians to three significant figures. ΜΕ ΑΣΦ vec (t)=0.3 cos (4.95t) + 0.101 sin (4.95t) Submit Previous Answers Request Answer × Incorrect; Try Again; 6 attempts remainingarrow_forwardPart A ■Review The uniform 150-lb stone (rectangular block) is being turned over on its side by pulling the vertical cable slowly upward until the stone begins to tip. (Figure 1) If it then falls freely (T = 0) from an essentially balanced at-rest position, determine the speed at which the corner A strikes the pad at B. The stone does not slip at its corner C as it falls. Suppose that height of the stone is L = 1.2 ft. Express your answer to three significant figures and include the appropriate units. ? ft VA 10.76 S Submit Previous Answers Request Answer × Incorrect; Try Again; 6 attempts remainingarrow_forwardConsider the circuit shown in the figure. The battery has emf ε = 69 volts and negligible internal resistance. The inductance is L = 0.4 H and the resistances are R 1 = 12 Ω and R 2 = 9.0 Ω. Initially the switch S is open and no currents flow. Then the switch is closed. After leaving the switch closed for a very long time, it is opened again. Just after it is opened, what is the current in R 1?arrow_forward
- A capacitor with a capacitance of C = 5.95×10−5 F is charged by connecting it to a 12.5 −V battery. The capacitor is then disconnected from the battery and connected across an inductor with an inductance of L = 1.55 H . At the time 2.35×10−2 s after the connection to the inductor is made, what is the current in the inductor? At that time, how much electrical energy is stored in the inductor?arrow_forwardCan someone help me with this question. Thanks.arrow_forwardCan someone help me with this question. Thanks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Polarization of Light: circularly polarized, linearly polarized, unpolarized light.; Author: Physics Videos by Eugene Khutoryansky;https://www.youtube.com/watch?v=8YkfEft4p-w;License: Standard YouTube License, CC-BY