
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN: 9781133104261
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 52P
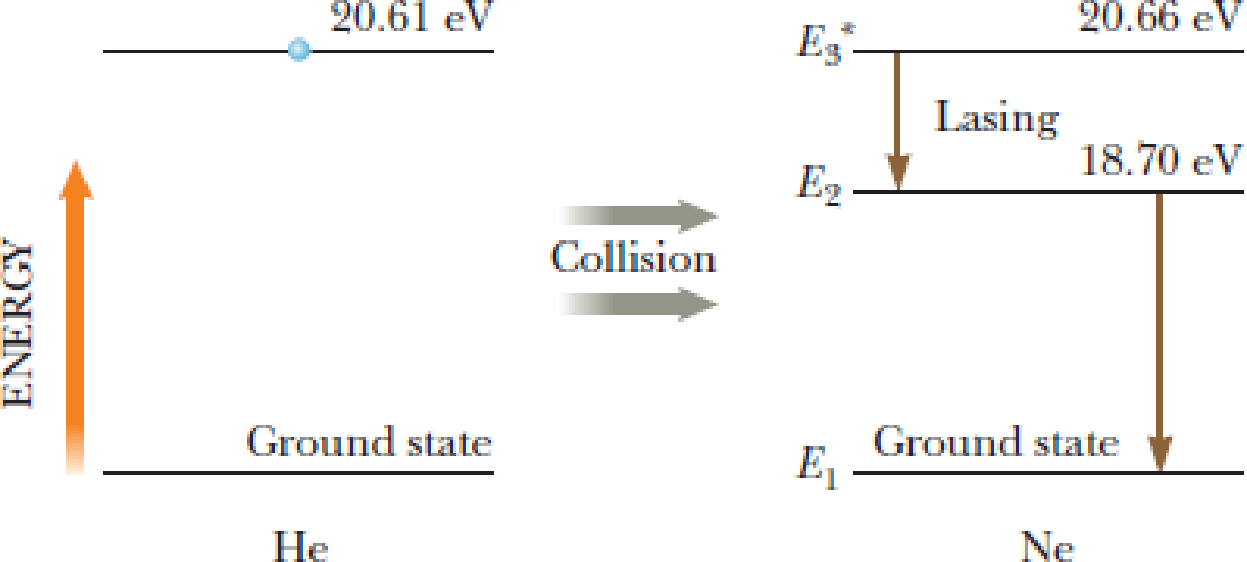
Figure P24.52 shows portions of the energy-level diagrams of the helium and neon atoms. An electrical discharge excites the He atom from its ground state (arbitrarily assigned the energy E1 = 0) to its excited state of 20.61 eV. The excited He atom collides with a Ne atom in its ground state and excites this atom to the state at 20.66 eV. Lasing action takes place for electron transitions from E3* to E2 in the Ne atoms. From the data in the figure, show that the wavelength of the red He–Ne laser light is approximately 633 nm.
Figure P24.52
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Lab 8 Part 3 PHET Wave Interface simulation.
I am having trouble with this part of the lab.
Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?
Hi,
I have canceled, why did you charge me again?
Chapter 24 Solutions
Principles of Physics: A Calculus-Based Text
Ch. 24.1 - Prob. 24.1QQCh. 24.4 - Prob. 24.2QQCh. 24.4 - Prob. 24.3QQCh. 24.4 - Prob. 24.4QQCh. 24.6 - Prob. 24.5QQCh. 24.6 - Prob. 24.6QQCh. 24.7 - Prob. 24.7QQCh. 24 - Prob. 1OQCh. 24 - Prob. 2OQCh. 24 - Prob. 3OQ
Ch. 24 - If plane polarized light is sent through two...Ch. 24 - Prob. 5OQCh. 24 - Prob. 6OQCh. 24 - Prob. 7OQCh. 24 - Prob. 9OQCh. 24 - Prob. 10OQCh. 24 - Prob. 11OQCh. 24 - Consider an electromagnetic wave traveling in the...Ch. 24 - Prob. 1CQCh. 24 - Prob. 2CQCh. 24 - Prob. 3CQCh. 24 - Prob. 4CQCh. 24 - Prob. 5CQCh. 24 - Prob. 6CQCh. 24 - Prob. 7CQCh. 24 - Prob. 8CQCh. 24 - Prob. 9CQCh. 24 - Prob. 10CQCh. 24 - Prob. 11CQCh. 24 - Prob. 12CQCh. 24 - Prob. 1PCh. 24 - Prob. 2PCh. 24 - Prob. 3PCh. 24 - A 1.05-H inductor is connected in series with a...Ch. 24 - Prob. 5PCh. 24 - Prob. 6PCh. 24 - Prob. 7PCh. 24 - An electron moves through a uniform electric field...Ch. 24 - Prob. 9PCh. 24 - Prob. 10PCh. 24 - Prob. 11PCh. 24 - Prob. 12PCh. 24 - Figure P24.13 shows a plane electromagnetic...Ch. 24 - Prob. 14PCh. 24 - Review. A microwave oven is powered by a...Ch. 24 - Prob. 16PCh. 24 - A physicist drives through a stop light. When he...Ch. 24 - Prob. 18PCh. 24 - Prob. 19PCh. 24 - A light source recedes from an observer with a...Ch. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - Prob. 25PCh. 24 - Prob. 26PCh. 24 - Prob. 27PCh. 24 - Prob. 28PCh. 24 - Prob. 29PCh. 24 - Prob. 30PCh. 24 - Prob. 31PCh. 24 - Prob. 32PCh. 24 - Prob. 33PCh. 24 - Prob. 34PCh. 24 - Prob. 35PCh. 24 - Prob. 36PCh. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - Prob. 39PCh. 24 - Prob. 40PCh. 24 - Prob. 41PCh. 24 - Prob. 42PCh. 24 - Prob. 43PCh. 24 - Prob. 44PCh. 24 - Prob. 45PCh. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - Prob. 48PCh. 24 - You use a sequence of ideal polarizing filters,...Ch. 24 - Prob. 50PCh. 24 - Prob. 51PCh. 24 - Figure P24.52 shows portions of the energy-level...Ch. 24 - Prob. 53PCh. 24 - Prob. 54PCh. 24 - Prob. 55PCh. 24 - Prob. 56PCh. 24 - Prob. 57PCh. 24 - Prob. 58PCh. 24 - Prob. 59PCh. 24 - Prob. 60PCh. 24 - Prob. 61PCh. 24 - Prob. 62PCh. 24 - A dish antenna having a diameter of 20.0 m...Ch. 24 - Prob. 65PCh. 24 - Prob. 66PCh. 24 - Prob. 67PCh. 24 - Prob. 68PCh. 24 - Prob. 69PCh. 24 - Prob. 70PCh. 24 - Prob. 71PCh. 24 - A microwave source produces pulses of 20.0-GHz...Ch. 24 - A linearly polarized microwave of wavelength 1.50...Ch. 24 - Prob. 74PCh. 24 - Prob. 75P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forwardA planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forward
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
Electric Fields: Crash Course Physics #26; Author: CrashCourse;https://www.youtube.com/watch?v=mdulzEfQXDE;License: Standard YouTube License, CC-BY