
(a)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.1P
The aldol product that formed by the given compound is
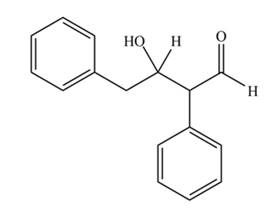
Explanation of Solution
The aldol product that formed by the given compound is shown below.
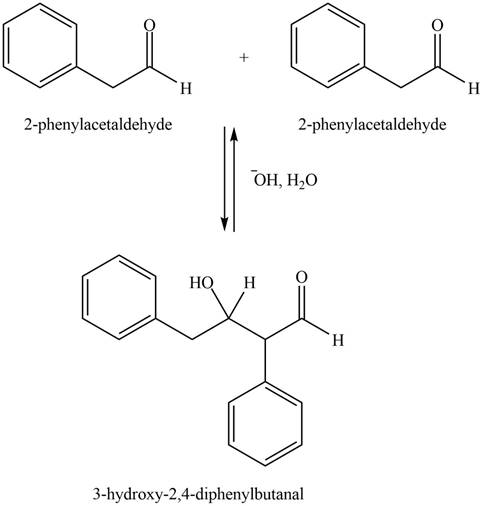
Figure 1
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(b)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
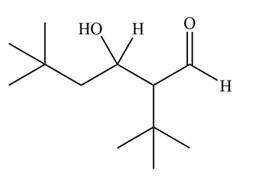
Explanation of Solution
The aldol product that formed by the given compound is shown below.
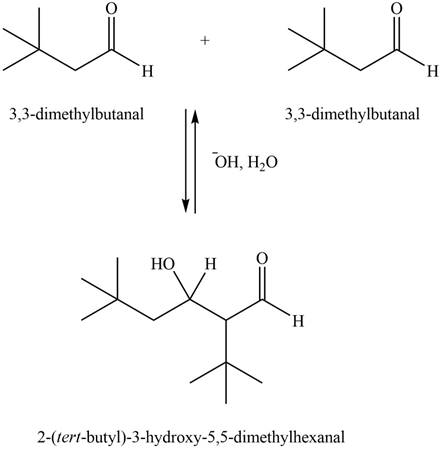
Figure 2
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(c)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
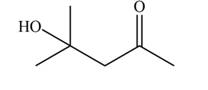
Explanation of Solution
The aldol product that formed by the given compound is shown below.
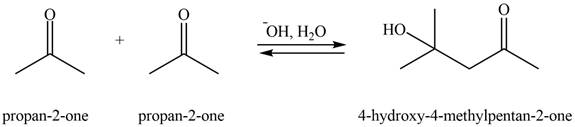
Figure 3
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(d)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
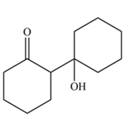
Explanation of Solution
The aldol product that formed by the given compound is shown below.
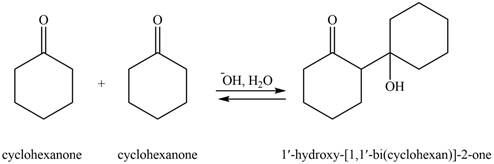
Figure 4
In this aldol reaction, one equivalent of cyclohexanone is treated with strong base that results in the formation of resonance-stabilized enolate ion. The second equivalent of cyclohexanone is then reacts with enolate ion. After that, hydrolysis of the intermediate compound takes place that results the formation of the desired product,
The aldol product that formed by the given compound is
Want to see more full solutions like this?
Chapter 24 Solutions
PKG ORGANIC CHEMISTRY
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





