
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 23.9, Problem 15P
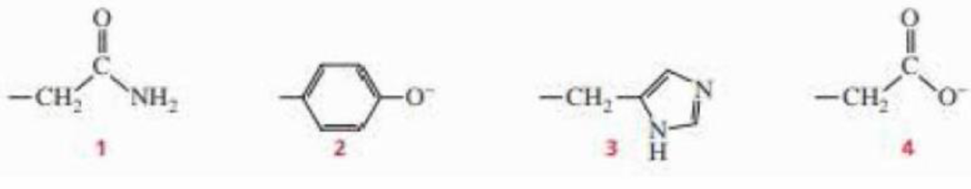
Which of the following amino acid side chains can help remove a proton from the α-carbon of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting
material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as
indicated by the boxes.
a.
NaOMe
H+
.CO,H
HO₂C
MeOH (excess)
MeOH
H3C
Br
يع
CH3
1. LiAlH4
2. H3O+
3. PBг3
H3C
1. Et-Li
2. H3O+
-CO₂Me
-CO₂Me
OH
CH3
CH3
ল
CH3
Predict the intermediate 1 and final product 2 of this organic reaction:
NaOMe
ག1, ད།་, -
+
H
You can draw 1 and 2 in any arrangement you like.
2
work up
Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge)
bonds at the chiral center.
Explanation
Check
Click and drag to start drawing a structure.
Х
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | P
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
Chapter 23 Solutions
Organic Chemistry
Ch. 23.2 - Compare each of the mechanisms listed here with...Ch. 23.2 - Prob. 3PCh. 23.2 - Prob. 4PCh. 23.3 - a. Draw the mechanism for the following reaction...Ch. 23.5 - Prob. 7PCh. 23.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 23.6 - Prob. 9PCh. 23.7 - Prob. 10PCh. 23.7 - Prob. 12PCh. 23.7 - Prob. 13P
Ch. 23.9 - Which of the following amino acid side chains can...Ch. 23.9 - Which of the following C-terminal peptide bonds is...Ch. 23.9 - Carboxypeptidase A has esterase activity as well...Ch. 23.9 - Arginine and lysine side chains fit into trypsins...Ch. 23.9 - Explain why serine proteases do not catalyze...Ch. 23.10 - If H2 18O is used in the hydrolysis reaction...Ch. 23.10 - When apples that have been cut are exposed to...Ch. 23.11 - Prob. 22PCh. 23.11 - The pHactivity profile for glucose-6-phosphate...Ch. 23.11 - Draw the pH-activity profile for an enzyme that...Ch. 23.12 - Prob. 25PCh. 23.12 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 23.12 - What advantage does the enzyme gain by forming an...Ch. 23.12 - Prob. 28PCh. 23.12 - Aldolase shows no activity if it is incubated with...Ch. 23 - Which of the following parameters would be...Ch. 23 - Prob. 30PCh. 23 - Prob. 31PCh. 23 - Prob. 32PCh. 23 - Indicate the type of catalysis that is occurring...Ch. 23 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 23 - Prob. 35PCh. 23 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 23 - there are two kinds of aldolases. Class I...Ch. 23 - Prob. 38PCh. 23 - The hydrolysis of the ester shown here is...Ch. 23 - Prob. 40PCh. 23 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 23 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 23 - Proof that an imine was formed between aldolase...Ch. 23 - Prob. 44PCh. 23 - a. Explain why the alkyl halide shown here reacts...Ch. 23 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY