
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.5, Problem 8P
Propose a mechanism for the Co2+ catalyzed hydrolysis of glycinamide.
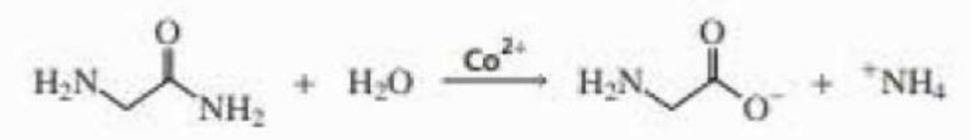
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write, in words three different reactions we can use to make an alcohol.
Draw the reduction mechanism for the reduction of the aldehyde.
NEED ONLY QUESTION 5 please
Chapter 23 Solutions
Organic Chemistry
Ch. 23.2 - Compare each of the mechanisms listed here with...Ch. 23.2 - Prob. 3PCh. 23.2 - Prob. 4PCh. 23.3 - a. Draw the mechanism for the following reaction...Ch. 23.5 - Prob. 7PCh. 23.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 23.6 - Prob. 9PCh. 23.7 - Prob. 10PCh. 23.7 - Prob. 12PCh. 23.7 - Prob. 13P
Ch. 23.9 - Which of the following amino acid side chains can...Ch. 23.9 - Which of the following C-terminal peptide bonds is...Ch. 23.9 - Carboxypeptidase A has esterase activity as well...Ch. 23.9 - Arginine and lysine side chains fit into trypsins...Ch. 23.9 - Explain why serine proteases do not catalyze...Ch. 23.10 - If H2 18O is used in the hydrolysis reaction...Ch. 23.10 - When apples that have been cut are exposed to...Ch. 23.11 - Prob. 22PCh. 23.11 - The pHactivity profile for glucose-6-phosphate...Ch. 23.11 - Draw the pH-activity profile for an enzyme that...Ch. 23.12 - Prob. 25PCh. 23.12 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 23.12 - What advantage does the enzyme gain by forming an...Ch. 23.12 - Prob. 28PCh. 23.12 - Aldolase shows no activity if it is incubated with...Ch. 23 - Which of the following parameters would be...Ch. 23 - Prob. 30PCh. 23 - Prob. 31PCh. 23 - Prob. 32PCh. 23 - Indicate the type of catalysis that is occurring...Ch. 23 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 23 - Prob. 35PCh. 23 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 23 - there are two kinds of aldolases. Class I...Ch. 23 - Prob. 38PCh. 23 - The hydrolysis of the ester shown here is...Ch. 23 - Prob. 40PCh. 23 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 23 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 23 - Proof that an imine was formed between aldolase...Ch. 23 - Prob. 44PCh. 23 - a. Explain why the alkyl halide shown here reacts...Ch. 23 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill-in-the molecules for the oxidation or reduction of the starting alcohol.arrow_forwardName the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forward
- While noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forwardWhat the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forward
- Which of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forwardplease answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forward
- Plan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forwardDraw the mechanism to make the alcohol 1-hexanol. Please use arrows.arrow_forwardAnswer the followings: 1-What is the difference(s) between DNA and RNA: a- Structure: b- Function: c- Types: 2-What is the meaning of: a- Replication b- Transcription c- Translation 3- Show the base pair connection (hydrogen bond) in DNA and RNAarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY