
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134649771
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.3, Problem 10P
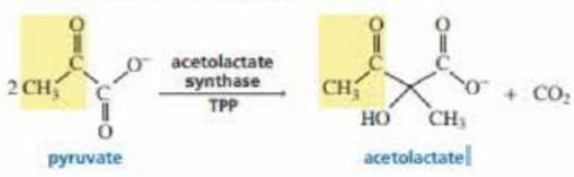
Acetolactate synthase transfers the acyl group of pyruvate to α-ketobutyrate. This is the first step in the biosynthesis of the amino acid isoleucine. Propose a mechanism for this reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
man Campus Depa
(a) Draw the three products (constitutional isomers) obtained when 2-methyl-3-hexene reacts with water and
a trace of H2SO4. Hint: one product forms as the result of a 1,2-hydride shift. (1.5 pts)
This is the acid-catalyzed alkene hydration reaction.
Chapter 23 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
Ch. 23.1 - Prob. 2PCh. 23.1 - Prob. 3PCh. 23.2 - How many conjugated double bonds are there in a....Ch. 23.2 - Instead of adding to the 4a position and...Ch. 23.2 - Prob. 7PCh. 23.3 - Prob. 8PCh. 23.3 - Acetolactate synthase is another TPP-requiring...Ch. 23.3 - Acetolactate synthase transfers the acyl group of...Ch. 23.3 - Prob. 12PCh. 23.5 - Which compound is more easily decarboxylated?
Ch. 23.5 - Prob. 14PCh. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - The enzyme that catalyzes the C C bond cleavage...Ch. 23.5 - Propose a mechanism for the ,-elimination reaction...Ch. 23.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 23.6 - Prob. 20PCh. 23.7 - How do the structure of tetrahydrofolate and...Ch. 23.7 - What is the source of the methyl group in...Ch. 23.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 23 - How does the metal ion in carboxypeptidase A...Ch. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - For each of the following reactions, name both the...Ch. 23 - Prob. 27PCh. 23 - When transaminated, the three branched-chain amino...Ch. 23 - What acyl groups have we seen transferred by...Ch. 23 - Propose a mechanism for the following reaction:Ch. 23 - Draw the products of the following reaction, where...Ch. 23 - When UMP is dissolved in T2O, exchange of T for H...Ch. 23 - Dehydratase is a PLP-requiring enzyme that...Ch. 23 - In addition to the reaction mentioned in Section...Ch. 23 - PLP can catalyze both ,-elimination reactions...Ch. 23 - The glycine cleavage system is a group of four...Ch. 23 - Prob. 37PCh. 23 - FADH2 reduces , -unsaturated thioesters to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (6 pts - 2 pts each part) Although we focused our discussion on hydrogen light emission, all elements have distinctive emission spectra. Sodium (Na) is famous for its spectrum being dominated by two yellow emission lines at 589.0 and 589.6 nm, respectively. These lines result from electrons relaxing to the 3s subshell. a. What is the photon energy (in J) for one of these emission lines? Show your work. b. To what electronic transition in hydrogen is this photon energy closest to? Justify your answer-you shouldn't need to do numerical calculations. c. Consider the 3s subshell energy for Na - use 0 eV as the reference point for n=∞. What is the energy of the subshell that the electron relaxes from? Choose the same emission line that you did for part (a) and show your work.arrow_forwardNonearrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception to the general ionization energy (IE) trend. For the two elements involved, answer the following questions. Be sure to cite sources for all physical data that you use. a. (2 pts) Identify the two elements and write their electronic configurations. b. (2 pts) Based on their configurations, propose a reason for the IE trend exception. c. (5 pts) Calculate effective nuclear charges for the last electron in each element and the Allred-Rochow electronegativity values for the two elements. Can any of these values explain the IE trend exception? Explain how (not) - include a description of how IE relates to electronegativity.arrow_forward
- Please explain thoroughly and provide steps to draw.arrow_forwardAs you can see in the picture, the instrument uses a Xe source. Given that the instrument is capable of measuring from 200-800nm, if Xe was not used, what other source(s) could be used? Refer to figure 7-3. How many monochrometers does this instrument have? Why? Trace the light as it goes from the Xenon lamp all the way to the circle just slightly to the right and a little bit down from S4. What do you think that circle is? In class we talked about many types of these, which kind do you think this one is for a fluorimeter? Why? Explain. What is/are some strategy(ies) that this instrument has for dealing with noise that you see present in the optics diagram? Why does a fluorescence cuvette have to be clear on four sides?arrow_forwardProvide steps and thoroughly solve.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY