
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134649771
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 35P
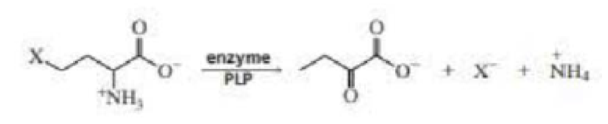
PLP can catalyze both α,β-elimination reactions (Problem 33) and β,γ-elimination reactions. Propose a mechanism for the following PLP-catalyzed β,γ-elimination reaction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Histamine (below structure) is a signal molecule involved in immune response and is
a neurotransmitter. Histamine features imidazole ring which is an aromatic heterocycle.
Please answer the following questions regarding Histamine.
b
a
HN
=N
C
NH2
a. Determine hybridization of each N atom (s, p, sp, sp², sp³, etc.) in histamine
N-a hybridization:
N-b hybridization:
N-c hybridization:
b. Determine what atomic orbitals (s, p, sp, sp², sp³, etc.) of the lone pair of each N
atom resided in
N-a hybridization:
N-b hybridization:
N-c hybridization:
None
29. Use frontier orbital analysis (HOMO-LUMO interactions) to decide whether the following
dimerization is 1) thermally allowed or forbidden and 2) photochemically allowed or
forbidden.
+
Chapter 23 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
Ch. 23.1 - Prob. 2PCh. 23.1 - Prob. 3PCh. 23.2 - How many conjugated double bonds are there in a....Ch. 23.2 - Instead of adding to the 4a position and...Ch. 23.2 - Prob. 7PCh. 23.3 - Prob. 8PCh. 23.3 - Acetolactate synthase is another TPP-requiring...Ch. 23.3 - Acetolactate synthase transfers the acyl group of...Ch. 23.3 - Prob. 12PCh. 23.5 - Which compound is more easily decarboxylated?
Ch. 23.5 - Prob. 14PCh. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - The enzyme that catalyzes the C C bond cleavage...Ch. 23.5 - Propose a mechanism for the ,-elimination reaction...Ch. 23.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 23.6 - Prob. 20PCh. 23.7 - How do the structure of tetrahydrofolate and...Ch. 23.7 - What is the source of the methyl group in...Ch. 23.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 23 - How does the metal ion in carboxypeptidase A...Ch. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - For each of the following reactions, name both the...Ch. 23 - Prob. 27PCh. 23 - When transaminated, the three branched-chain amino...Ch. 23 - What acyl groups have we seen transferred by...Ch. 23 - Propose a mechanism for the following reaction:Ch. 23 - Draw the products of the following reaction, where...Ch. 23 - When UMP is dissolved in T2O, exchange of T for H...Ch. 23 - Dehydratase is a PLP-requiring enzyme that...Ch. 23 - In addition to the reaction mentioned in Section...Ch. 23 - PLP can catalyze both ,-elimination reactions...Ch. 23 - The glycine cleavage system is a group of four...Ch. 23 - Prob. 37PCh. 23 - FADH2 reduces , -unsaturated thioesters to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 30.0 mL of 0.10 mol/L iron sulfate and 20.0 mL of 0.05 mol/L of silver nitrate solutions are mixed together. Justify if any precipitate would formarrow_forwardDoes the carbonyl group first react with the ethylene glycol, in an intermolecular reaction, or with the end alcohol, in an intramolecular reaction, to form a hemiacetal? Why does it react with the alcohol it does first rather than the other one? Please do not use an AI answer.arrow_forwardThe number of noncyclic isomers that have the composition C4H8Owith the O as part of an OH group, counting a pair of stereoisomers as1, is A. 8; B. 6; C. 9; D. 5; E. None of the other answers is correct.arrow_forward
- Nonearrow_forwardThe number of carbon skeletons that have 8 carbons, one of which istertiary is A. 7; B. More than 7; C. 6; D. 5; E. 4arrow_forwardThe azide ion is N3^-. In addition to the ionic charge, it’s three mostimportant contributing structures also have formal charges. The totalnumber of π bonds in these three contributing structures isA. 6; B. 12; C. 3; D. 9; E. None of the other answers is correct.arrow_forward
- The sum of the numerals in the name of the compoundis A. None of the other answers is correct.; B. 11;C. 6; D. 8; E. 5.arrow_forwardA compound has a six carbon ring with three double bonds. Attachedto the ring is a three carbon chain with a triple bond and a two carbonchain with two bromines attached. The number of hydrogens in a molecule of this compound is A. 10; B. 12; C. 14; D. 13; E. None of the other answers is correct.arrow_forwardCan you help me? I can't seem to understand the handwriting for the five problems, and I want to be able to solve them and practice. If you'd like to give me steps, please do so to make it easier understand.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License