
Concept explainers
(a)
Interpretation:
The structure of N-isopropylaniline is to be drawn.
Concept introduction:
Answer to Problem 23.1P
The structure of N-isopropylaniline is shown below.
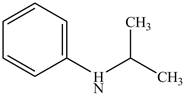
Explanation of Solution
The name N-isopropylaniline indicates that it consists of the structure of aniline compound. One hydrogen atom of
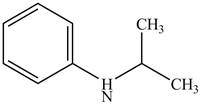
Figure 1
The structure of N-isopropylaniline is shown in Figure 1.
(b)
Interpretation:
The structure of tert-butylamine is to be drawn.
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of tert-butylamine is shown below.
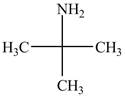
Explanation of Solution
The name tert-butylamine indicates that it consist of tert-butyl group. The tert-butyl group is attached to an amine group. The structure of tert-butylamine is shown below.
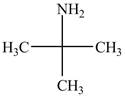
Figure 2
The structure of tert-butylamine is shown in Figure 2.
(c)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
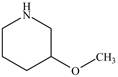
Explanation of Solution
The name
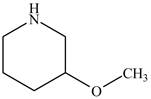
Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
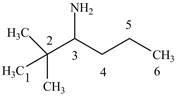
Explanation of Solution
The name
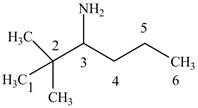
Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
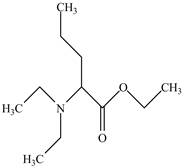
Explanation of Solution
The name
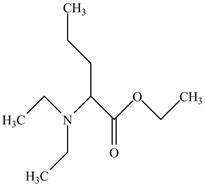
Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
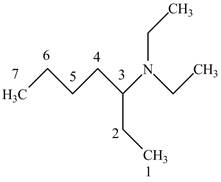
Explanation of Solution
The name
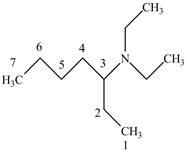
Figure 6
The structure of
Want to see more full solutions like this?
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY
- Can u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



