
Concept explainers
(a)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
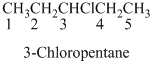
If the molecules having
The given compound is an alcohol
Example is given below
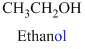
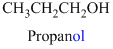
The given compound is an acid (
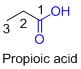
The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
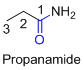
If the molecule is ester,
Esters end with “ate”
Example
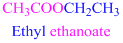
The given compound is an
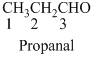
The given compound is an aldehyde (
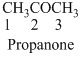
The given compound is an aldehyde (
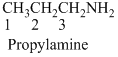
(a)
Answer to Problem 23.19QP
Answer
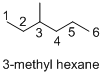
- (1) Structure of the given organic compounds is shown below (a).
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 3-methyl hexane.

Parent chain is identified and numbering is given for the compound. According to the name, third carbon is bearing one methyl group and the given name is hexane which clearly indicates that the compound having six carbon atoms in the molecule. According to the name, the structure of the molecule is given above.
(b)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (4) Name the parent alkane (long alkyl chain)
- (5) Number the carbon
- (6) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
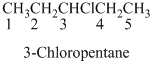
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
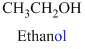
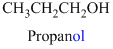
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
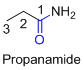
If the molecule is ester,
Esters end with “ate”
Example
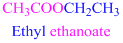
The given compound is an aldehyde (
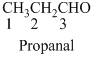
The given compound is an aldehyde (
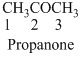
The given compound is an aldehyde (
(b)
Answer to Problem 23.19QP
Answer
Structure of the given organic compounds is shown below (b).
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 2,3-dimethylpentane
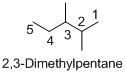
Parent chain is identified and numbering is given for the compound. According to the name, second and third carbon is bearing methyl group and the parent name of the molecule is pentane which clearly indicates that the compound having five carbon atoms in the parent. According to the name, the structure of the molecule is given above.
(c)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (7) Name the parent alkane (long alkyl chain)
- (8) Number the carbon
- (9) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
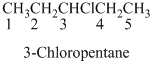
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
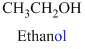
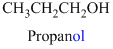
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
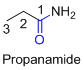
If the molecule is ester,
Esters end with “ate”
Example
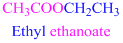
The given compound is an aldehyde (
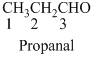
The given compound is an aldehyde (
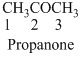
The given compound is an aldehyde (
(c)
Answer to Problem 23.19QP
Structure of the given organic compounds is shown below (c).
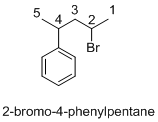
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 2-bromo-4-phenylpentane.

Parent chain is identified and numbering is given for the compound. According to the name, second carbon is bearing bromine and fourth carbon is bearing phenyl ring and the parent name of the molecule is pentane which clearly indicates that the compound having five carbon atoms in the parent. According to the name, the structure of the molecule is given above.
(d)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (10) Name the parent alkane (long alkyl chain)
- (11) Number the carbon
- (12) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
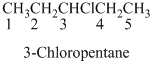
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
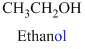
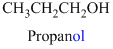
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
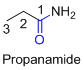
If the molecule is ester,
Esters end with “ate”
Example
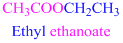
The given compound is an aldehyde (
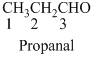
The given compound is an aldehyde (
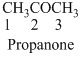
The given compound is an aldehyde (
(d)
Answer to Problem 23.19QP
Answer
Structure of the given organic compounds is shown below (d).

Explanation of Solution
To find: The structure of the given organic molecule.
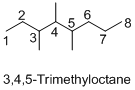
Name of the given organic compounds is 3,4,5-trimethyloctane.

Parent chain is identified and numbering is given for the compound. According to the name, third, fourth and fifth carbon is bearing methyl group and the parent name of the molecule is octane which clearly indicates that the compound having eight carbon atoms in the parent. According to the name, the structure of the molecule is given above.
Want to see more full solutions like this?
Chapter 23 Solutions
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
- pls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


