
(a)
Interpretation:
A portion of the polymer made from tetrachloroethylene needs to be drawn.
Concept Introduction:
A polymer is a long chain consists of large number of monomer units. In a polymer, the monomers are linked to each other in a continuous or repetitive manner. These monomer units are linked to each other either through the formation of peptide linkage,glycosidic linkage or by removal of any moiety such as a water molecule.Polyvinyl chloride, Bakelite and polystyrene are some of the examples of the
(a)
Answer to Problem 1QAP
A portion of polymer made from tetrachloroethylene is as follows:
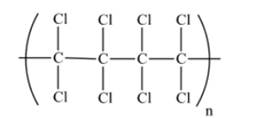
Explanation of Solution
Tetrachloroethylene polymer consists of number of monomer unit of 1,1,2,2-tetrachloroethylene.
The structure of tetrachloroethylene or monomer unit of the polymer is as follows:
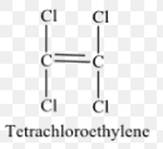
The polymer is formed after breaking of C-C double bonds, the two terminal carbon atoms of 1 tetrachloroethylene unit form bond with 2 terminal carbons of two other tetrachloroethyleneunits. To form a polymer, n numbers of such tetrachloroethyleneunits combined in the same way.
A portion of this polymer is shown as follows:
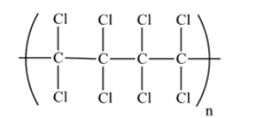
(b)
Interpretation:
The molar mass of the polymer needs to be calculated if it contains
Concept Introduction:
A polymer is a long chain consists of large number of monomer units. In a polymer, the monomers are linked to each other in a continuous or repetitive manner. These monomer units are linked to each other either through the formation of peptide linkage, glycosidic linkage or by removal of any moiety such as a water molecule. Polyvinyl chloride, Bakelite and polystyrene are some of the examples of the polymers.
Tetrachloroethylene polymer consists of number of monomer unit of 1,1,2,2-tetrachloroethylene.
(b)
Answer to Problem 1QAP
The molar mass of complete polymer is
Explanation of Solution
Molar mass of 1 monomer unit of 1,1,2,2-tetrachloroethylene is 168 g /mol. This is mass of 1 molecule of the 1,1,2,2-tetrachloroethylene.
If the polymer contains
Thus, molar mass of complete polymer is
(c)
Interpretation:
The mass percent of C and Cl in the polymer needs to be determined.
Concept Introduction:
Mass percent of an atom present in the sample can be determined by dividing mass of atom present in the monomer to the overall mass of the monomer unit and multiplying the overall result with 100%.
For example, the mass percent of x g of an atom present in the y g of monomer unit can be determined as:
(c)
Answer to Problem 1QAP
Mass percent of C in polymer is 14.28 %.
Mass percent of Cl in polymer is 83.33 %.
Explanation of Solution
The molar mass of the 1 tetrachloroethylene unit is 168 g/mol.
There are 2 C atoms in a monomer unit of tetrachloroethylene.
Now, molar mass of C atom in a monomer will be:
Putting the values,
Thus, mass percent of C in polymer is 14.28 %.
The number of Cl atoms in the monomer unit of tetrachloroethylene is 4.
Molar mass of Cl in a monomer will be:
Mass percent of Cl can be calculated as follows:
Putting the values,
Thus, mass percent of Cl in the polymer is 83.33 %
Want to see more full solutions like this?
Chapter 23 Solutions
OWLV2 FOR MASTERTON/HURLEY'S CHEMISTRY:
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


