
(a)
Interpretation:
The pH at which the zwitter ion [Z] and the cationic form [C+] of the amino acid alanine become equal needs to be calculated.
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main
functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value. - Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 2.29
Explanation of Solution
The given amino acid is alanine which has the following structure:
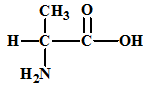
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
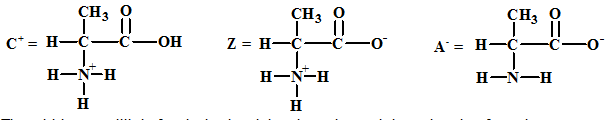
The acid-base equilibria for alanine involving the cation and the zwitter ion forms is:
(b)
Interpretation:
The pH at which the zwitter ion [Z] and the anionic form [A-] of the amino acid alanine become equal needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 9.74
Explanation of Solution
The given amino acid is alanine which has the following structure:
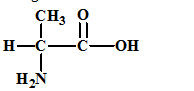
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
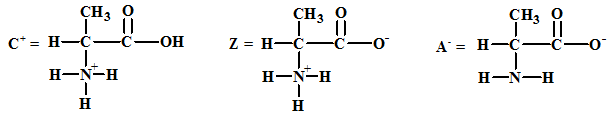
The acid-base equilibria for alanine involving the anion and the zwitter ion forms is:
(c)
Interpretation:
The pH at the isoelectric point needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 6.02
Explanation of Solution
For alanine at the isoelectric point:
Want to see more full solutions like this?
Chapter 23 Solutions
OWLV2 FOR MASTERTON/HURLEY'S CHEMISTRY:
- presented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
- 8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forwardо но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forward
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

