
a)
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
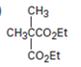
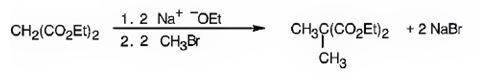
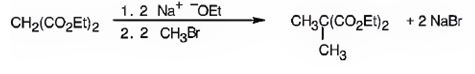
The compound shown can be prepared by using malonic ester synthesis.
Explanation of Solution
The compound shown is a derivative of carboxylic acid. Hence it can be prepared using malonic ester synthesis. The acid has two methyl groups attached to the carbon adjacent to ester groups. It can be prepared by replacing the two hydrogens on the active methylene group of malonic ester by two methyl groups. This is achieved by treating the ester with two equivalents of sodium ethoxide and two equivalents of methyl bromide.
The compound shown can be prepared by using malonic ester synthesis.
b)
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
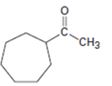
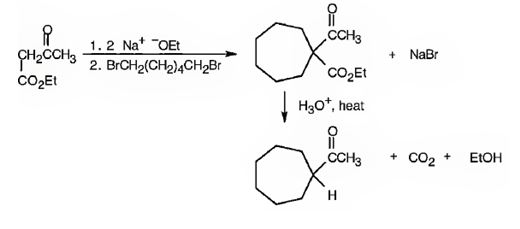
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
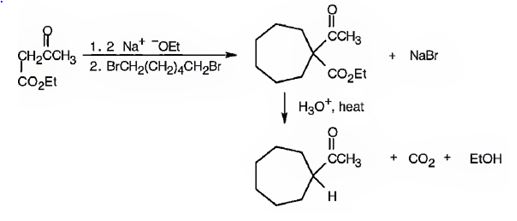
Explanation of Solution
: The compound is a methyl ketone. Hence it can be prepared using aceto acetic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of acetoacetic ester to yield the enolate anion. The nucleophilic attack of the anion on 1,6- dibromohexane displaces the bromide ion to produce a α- substituted acetoacetic ester. The second acidic hydrogen of the ester is then removed by another ethoxide ion which is followed by the nucleophilic attack of the anion on the other carbon bearing bromine to produce a cyclic ester. Upon treating with aqueous acids the ester group gets hydrolyzed to give a β- ketocarboxylic acid. The ketocarboxylic acid eliminates a CO2 molecule on heating to yield methyl cycloheptyl ketone.
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
c)
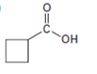
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
The compound shown can be prepared by using malonic ester synthesis.
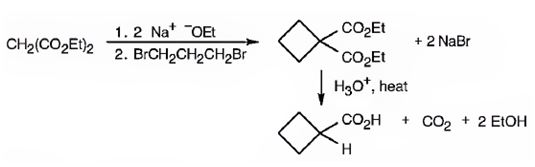
Explanation of Solution
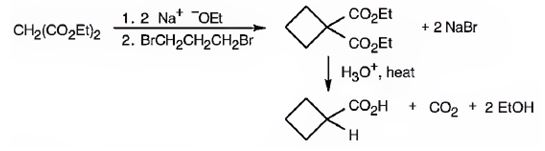
The compound shown is a carboxylic acid. Hence it can be prepared using malonic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of malonic ester to yield the enolate anion. The nucleophilic attack of the anion on 1,3- dibromopropane displaces the bromide ion to produce a α- substituted malonic ester. The second acidic hydrogen of the ester is then removed by another ethoxide ion which is followed by the nucleophilic attack of the anion on the other carbon bearing bromine to produce a cyclic diester. Upon treating with aqueous acids the ester groups get hydrolyzed to give a dicarboxylic acid. The dicarboxylic acid eliminates a CO2 molecule on heating to yield cyclobutylcarboxylic acid.
The compound shown can be prepared by using malonic ester synthesis.
d)
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
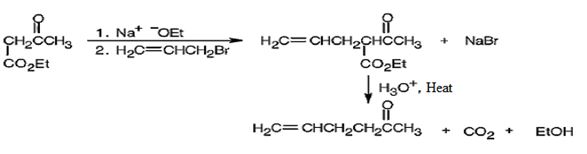
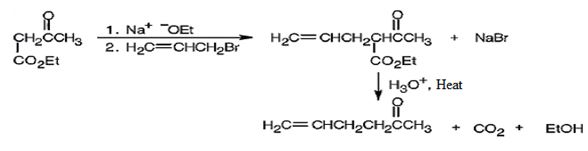
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
Explanation of Solution
The compound is a methyl ketone. Hence it can be prepared using aceto acetic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of acetoacetic ester to yield the enolate anion. The nucleophilic attack of the anion on allyl bromide displaces the bromide ion to produce α- allylsubstituted acetoacetic ester. Upon treating with aqueous acids the ester group gets hydrolyzed to give a β- ketocarboxylic acid. The ketocarboxylic acid eliminates a CO2 molecule on heating to yield hex-5-ene-2-one.
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY W/OWL
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


