
(a)
Interpretation:
For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 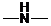 and
and 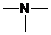 groups are attached to the parent carbon, they are called primary, secondary and tertiary
groups are attached to the parent carbon, they are called primary, secondary and tertiary
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(b)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 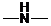 and
and 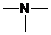 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(c)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 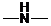 and
and 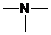 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(d)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 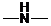 and
and 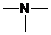 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(e)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 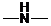 and
and 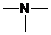 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEM PRINT STUDY GDE & SSM
- О δα HO- H -Br δα HO-- + + -Br [B] 8+ HO- -Br δα नarrow_forward1/2 - 51% + » GAY Organic Reactions Assignment /26 Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted. H3C 1. 2. CH3 A Acid OH Type of Reaction: NH Type of Reaction: + H₂O Catalyst + HBr 3. Type of Reaction: H3C 4. Type Reaction: 5. H3C CH2 + H2O OH + [0] CH3 Type of Reaction: 6. OH CH3 HO CH3 + Type of Reaction: 7. Type of Reaction: + [H]arrow_forwardhumbnai Concentration Terms[1].pdf ox + New Home Edit Sign in Comment Convert Page Fill & Sign Protect Tools Batch +WPS A Free Trial Share Inter Concreting Concentration forms. Hydrogen peroxide is a powerful oxidizing agent wed in concentrated solution in rocket fuels and in dilute solution as a hair bleach. An aqueous sulation of H2O2 is 30% by mass and has density of #liligime calculat the Ⓒmolality ⑥mole fraction of molarity. 20 9. B. A sample of Commercial Concentrated hydrochloric ETarrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forward
- Draw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forwardChoose the right answerarrow_forward8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forward
- Choose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





