
(a)
Interpretation: The formation of azo dyes has to be found via an azo coupling reaction by using the given set of more activated ring.
Concept Introduction:
Aryldiazonium salts on coupling with an activated
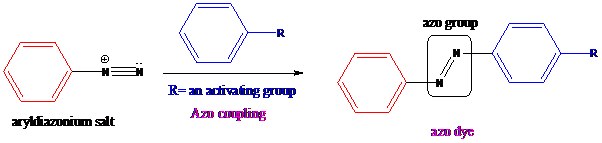
To find: Get the product of azo dye when diazonium salt formed from aniline is treated with aniline (a) via an azo coupling reaction
Determine the formation of diazonium slat

(b)
Interpretation: The formation of azo dyes has to be found via an azo coupling reaction by using the given set of more activated ring.
Concept Introduction:
Aryldiazonium salts on coupling with an activated aromatic ring give an azo dye which produces a deep color in the final product. This color is due to the extended conjugation in it. This reaction occurs by electrophilic substitution reaction. This method is called an azo coupling. The obtained final compound is known as azo dyes.

To find: Get the product of azo dye when diazonium salt formed from aniline is treated with aniline (b) via an azo coupling reaction
Determine the formation of diazonium slat

(c)
Interpretation: The formation of azo dyes has to be found via an azo coupling reaction by using the given set of more activated ring.
Concept Introduction:
Aryldiazonium salts on coupling with an activated aromatic ring give an azo dye which produces a deep color in the final product. This color is due to the extended conjugation in it. This reaction occurs by electrophilic substitution reaction. This method is called an azo coupling. The obtained final compound is known as azo dyes.

To find: Get the product of azo dye when diazonium salt formed from aniline is treated with aniline (c) via an azo coupling reaction
Determine the formation of diazonium slat

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
KLEIN'S ORGANIC CHEMISTRY
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





