
To explain:
The structure of the pH scale and the importance of buffers to biological systems.
Introduction:
The pH scale is used to measure the alkalinity and the acidity of the solution. It ranges on a scale from 0-14. The pH of 7 is considered neutral, pH below 7 is considered acidic and pH above 7 is considered alkaline. The pH above 7 is considered alkaline due to the increase in the concentration of the hydroxide ions and pH below 7 is acidic due to the increase in the concentration of the hydrogen ions. The concentration of the hydrogen and hydroxide ions is maintained by the addition of buffers into the solution. The examples include carbonic acid, and acetic acid. The carbonic acid dissociates to form the hydrogen ions and the bicarbonate ions, acetic acid dissociated to form acetate ions and hydrogen ions and water consists of hydrogen and hydroxide ions. The examples of acidic solutions are lemon juice, vinegar, tomatoes and coffee and examples of alkaline solutions include milk of magnesia and ammonia.
Pictorial representation:
The structure of pH scale is well represented in Fig.1.
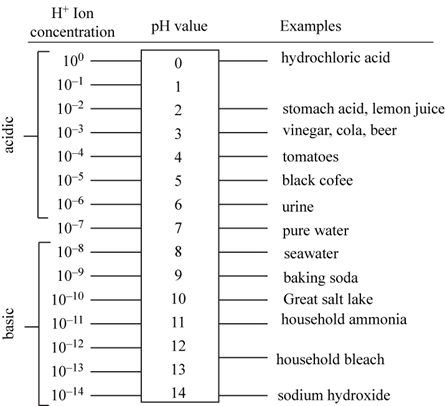
Fig.1: Structure of pH scale ranging from 0 to 14.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
HUMAN BIOLOGY + VIRTUAL PAC
- With reference to their absorption spectra of the oxy haemoglobin intact line) and deoxyhemoglobin (broken line) shown in Figure 2 below, how would you best explain the reason why there are differences in the major peaks of the spectra? Figure 2. SPECTRA OF OXYGENATED AND DEOXYGENATED HAEMOGLOBIN OBTAINED WITH THE RECORDING SPECTROPHOTOMETER 1.4 Abs < 0.8 06 0.4 400 420 440 460 480 500 520 540 560 580 600 nm 1. The difference in the spectra is due to a pH change in the deoxy-haemoglobin due to uptake of CO2- 2. There is more oxygen-carrying plasma in the oxy-haemoglobin sample. 3. The change in Mr due to oxygen binding causes the oxy haemoglobin to have a higher absorbance peak. 4. Oxy-haemoglobin is contaminated by carbaminohemoglobin, and therefore has a higher absorbance peak 5. Oxy-haemoglobin absorbs more light of blue wavelengths and less of red wavelengths than deoxy-haemoglobinarrow_forwardWhich ONE of the following is FALSE regarding haemoglobin? It has two alpha subunits and two beta subunits. The subunits are joined by disulphide bonds. Each subunit covalently binds a haem group. Conformational change in one subunit can be transmitted to another. There are many variant ("mutant") forms of haemoglobin that are not harmful.arrow_forwardWhich ONE of the following is FALSE regarding haemoglobin? It has two alpha subunits and two beta subunits. The subunits are joined by disulphide bonds. Each subunit covalently binds a haem group. Conformational change in one subunit can be transmitted to another. There are many variant ("mutant") forms of haemoglobin that are not harmful.arrow_forward
- During a routine medical check up of a healthy man it was found that his haematocrit value was highly unusual – value of 60%. What one of the options below is the most likely reason? He will have a diet high in iron. He is likely to be suffering from anaemia. He lives at high altitude. He has recently recovered from an accident where he lost a lot of blood. He has a very large body size.arrow_forwardExplain what age of culture is most likely to produce an endospore?arrow_forwardExplain why hot temperatures greater than 45 degrees celsius would not initiate the sporulation process in endospores?arrow_forward
- Endospore stain: Consider tube 2 of the 7-day bacillus culture. After is was heated, it was incubated for 24 hours then refrigerated. Do you think the cloudiness in this tube is due mostly to vegetative cells or to endospores? Explain your reasoningarrow_forwardReactunts C6H12O6 (Glucose) + 2NAD+ + 2ADP 2 Pyruvic acid + 2NADH + 2ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? GHz 06 (glucose) d. Which reactant is the electron acceptor? NAD e. Which of the products have been reduced? NADH f. Which of the products have been oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished? 3arrow_forwardThere is ________ the concept of global warming. Very strong evidence to support Some strong evidence to support Evidence both supporting and against Evidence againstarrow_forward
- How many types of reactions can an enzyme perform?arrow_forwardYour goal is to produce black seeds resistant to mold. So you make the same cross again (between a homozygous black seeded, mold susceptible parent and a homozygous white seeded and mold resistant parent), and, again, advance progeny by SSD to create 100 F10 generation plants. Based on the information you obtained from your first crossing experiment (Question #4), how many F10 plants would you expect to have black seeds and be resistant to mold? Assume that a toxin produced by the mold fungus has been isolated. Only mold resistant seeds will germinate in the presence of the toxin. Could you use this toxin screening procedure to have segregation distortion work in your favor in the F2 generation? Explain your answer. Info from Question 4 a. P Locus (Seed Color): Hypothesis: The null hypothesis (H₀) is that seed color is controlled by alleles at a single locus. Observed Data: Total white seeds: 45 (resistant plants) + 6 (susceptible plants) = 51 Total black seeds: 7 (resistant…arrow_forward10. Consider the following enzyme and its substrate where the "+" and "-" indicate cations and anions, respectively. Explain which of the following inhibitors could inhibit this enzyme? Which type of inhibitor would it be and why? (Video 5-2) Substrate Enzyme Potential inhibitorsarrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





