
Concept explainers
Interpretation:
The structure of the disaccharide trehalose based on the given information’s provided is to be identified.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
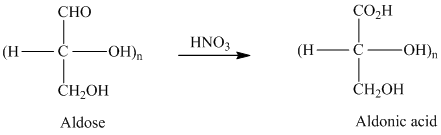
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
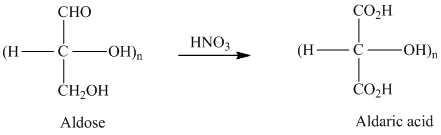
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 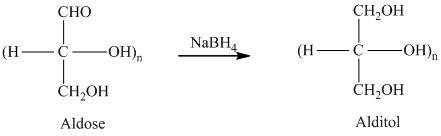
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 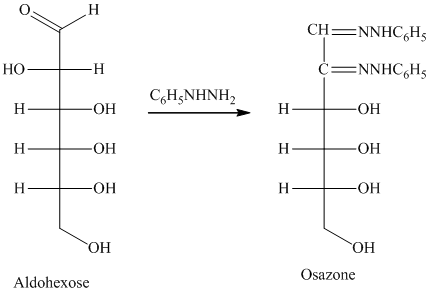
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧. Bromine water is an effective reagent that selectively oxidizes the
舧 Trehalose is a disaccharide found in yeasts, fungi, sea urchins, algae and insects. Its molecular formula is
舧 Sugars that react positively with Tollen’s reagent or Benedict’s solution are called reducing sugars, while those that do not react with Tollen’s reagent are called nonreducing sugars.
舧 The aldehyde group of an aldose reacts with three moles of phenylhydrazine to produce phenylosazone at
舧 Mutarotation is the change in values of specific rotation of each anomer while attaining equilibrium in an aqueous solution.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
- Classify each of the amino acids below. Note for advanced students: none of these amino acids are found in normal proteins. X CH2 H3N-CH-COOH3N-CH-COO- H3N-CH-COO CH2 CH3-C-CH3 CH2 NH3 N NH (Choose one) ▼ (Choose one) S CH2 OH (Choose one) ▼ + H3N-CH-COO¯ CH2 H3N CH COO H3N-CH-COO CH2 오오 CH CH3 CH2 + O C CH3 O= O_ (Choose one) (Choose one) ▼ (Choose one) Garrow_forwardAnother standard reference electrode is the standard calomel electrode: Hg2Cl2(s) (calomel) + 2e2 Hg() +2 Cl(aq) This electrode is usually constructed with saturated KCI to keep the Cl- concentration constant (similar to what we discussed with the Ag-AgCl electrode). Under these conditions the potential of this half-cell is 0.241 V. A measurement was taken by dipping a Cu wire and a saturated calomel electrode into a CuSO4 solution: saturated calomel electrode potentiometer copper wire CuSO4 a) Write the half reaction for the Cu electrode. b) Write the Nernst equation for the Cu electrode, which will include [Cu2+] c) If the voltage on the potentiometer reads 0.068 V, solve for [Cu²+].arrow_forward2. (Part B). Identify a sequence of FGI that prepares the Synthesis Target 2,4-dimethoxy- pentane. All carbons in the Synthesis Target must start as carbons in either ethyne, propyne or methanol. Hint: use your analysis of Product carbons' origins (Part A) to identify possible structure(s) of a precursor that can be converted to the Synthesis Target using one FGI. All carbons in the Synthesis Target must start as carbons in one of the three compounds below. H = -H H = -Me ethyne propyne Synthesis Target 2,4-dimethoxypentane MeOH methanol OMe OMe MeO. OMe C₂H₁₂O₂ Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forward
- Draw the skeletal ("line") structure of the smallest organic molecule that produces potassium 3-hydroxypropanoate when reacted with KOH. Click and drag to start drawing a structure. Sarrow_forwardDraw the skeleatal strucarrow_forward← Problem 14 of 31 Submit Draw the major product for this reaction. Ignore inorganic byproducts. 1. BH3-THF 2. H2O2, NaOHarrow_forward
- Classify each amino acid below as nonpolar, polar neutral, polar acidic, or polar basic.arrow_forwarddraw skeletal structures for the minor products of the reaction.arrow_forward1. Provide missing starting materials, reagents, products. If a product cannot be made, write NP (not possible) in the starting material box. C7H12O Ph HO H 1) 03-78 C 2) Me₂S + Ph .H OH + 2nd stereoisomer OH Ph D + enantiomer cat OsO 4 NMO H2O acetonearrow_forward
- Please note that it is correct and explains it rightly:Indicate the correct option. The proportion of O, C and H in the graphite oxide is:a) Constant, for the quantities of functional groups of acids, phenols, epoxy, etc. its constants.b) Depending on the preparation method, as much oxidant as the graphite is destroyed and it has less oxygen.c) Depends on the structure of the graphic being processed, whether it can be more tridimensional or with larger crystals, or with smaller crystals and with more edges.arrow_forwardCheck the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. ནང་་་ OH HO HO NH2 + NH3 O OIL H-C-CO CH3-CH O C=O COOH COOH + H2N C-H O H2N C H CH3-CH CH2 HO H3N O none of them 口 CH3 CH2 OH Хarrow_forwardWhat is the systematic name of the product P of this chemical reaction? 010 HO-CH2-CH2-C-OH ☐ + NaOH P+ H2Oarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





