
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
10th Edition
ISBN: 9781259972348
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 31P
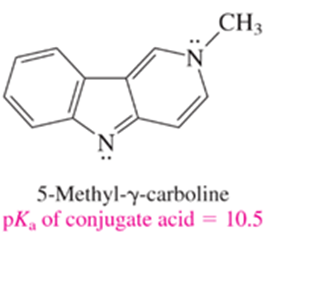
The compound shown is a somewhat stronger base than ammonia. Which nitrogen do you think is protonated when it is treated with an acid? Write a structural formula for the species that results.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
We learned four factors (ARIO) for comparing the relative acidity of compounds. When two of these factors are in
competition, the order of priority is the order in which these factors were covered ("atom" being the most important factor
and "orbital" being the least important). However, we also mentioned that there are exceptions to this order of priority.
Compare the two compounds and identify the exception.
OH
PK-4.75
SH
PK-10.6
5.
"Resonance" is more important than "atom" because the conjugate base of first compound is more stable than the
second.
"Atom" is more important than "resonance" because the conjugate base of first compound is more stable than the
second.
"Resonance" is more important than "atom" because the conjugate base of second compound is more stable than
the first.
"Atom" is more important than "resonance" because the conjugate base of second compound is more stable than
the first.
The relative fitnesses of three genotypes are WA/A= 1.0, WA/a = 0.7, and Wa/a =
0.3. If the population starts at the allele frequency p = 0.5, what is the value of p
in the next generation? (3 pts)
12pt v
Paragraph V
BIU AL
Identify the most acidic proton in the compound:
a
d
b
Оа
Ob
Ос
○ d
Chapter 22 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
Ch. 22.1 - Prob. 1PCh. 22.1 - Prob. 2PCh. 22.1 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.4 - Prob. 5PCh. 22.4 - Prob. 6PCh. 22.4 - Prob. 7PCh. 22.4 - Prob. 8PCh. 22.4 - Prob. 9PCh. 22.7 - Prob. 10P
Ch. 22.8 - Problem 22.11 Three of the following amines can be...Ch. 22.9 - Prob. 12PCh. 22.10 - Prob. 13PCh. 22.13 - Prob. 14PCh. 22.14 - Prob. 15PCh. 22.15 - Prob. 16PCh. 22.15 - Prob. 17PCh. 22.17 - Prob. 18PCh. 22.17 - Prob. 19PCh. 22.17 - Prob. 20PCh. 22.17 - Prob. 21PCh. 22.18 - Prob. 22PCh. 22 - Prob. 23PCh. 22 - Prob. 24PCh. 22 - Prob. 25PCh. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - Arrange the following compounds or anions in each...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - The compound shown is a somewhat stronger base...Ch. 22 - Prob. 32PCh. 22 - Prob. 33PCh. 22 - Prob. 34PCh. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - Write the structure of the product formed on...Ch. 22 - Prob. 38PCh. 22 - Prob. 39PCh. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - Devise efficient syntheses of each of the...Ch. 22 - Prob. 46PCh. 22 - Prob. 47PCh. 22 - Prob. 48PCh. 22 - Prob. 49PCh. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - Prob. 52PCh. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56DSPCh. 22 - Prob. 57DSPCh. 22 - Prob. 58DSPCh. 22 - Prob. 59DSPCh. 22 - Synthetic Applications of Enamines The formation...Ch. 22 - Prob. 61DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A Standard Reference Material is certified to contain 94.6 ppm of an organic contaminant in soil. Your analysis gives values of 98.6, 98.4, 97.2, 94.6, and 96.2. Do your results differ from the expected results at the 95% confidence interval?arrow_forwardThe percentage of an additive in gasoline was measured six times with the following results: 0.13, 0.12, 0.16, 0.17, 0.20, and 0.11%. Find the 95% confidence interval for the percentage of additive.arrow_forwardExplain why this data led Rayleigh to look for and to discover Ar.arrow_forward
- 5) Confidence interval. Berglund and Wichardt investigated the quantitative determination of Cr in high-alloy steels using a potentiometric titration of Cr(VI). Before the titration, samples of the steel were dissolved in acid and the chromium oxidized to Cr(VI) using peroxydisulfate. Shown here are the results (as %w/w Cr) for the analysis of a reference steel. 16.968, 16.922, 16.840, 16.883, 16.887, 16.977, 16.857, 16.728 Calculate the mean, the standard deviation, and the 95% confidence interval about the mean. What does this confidence interval mean?arrow_forwardIn the Nitrous Acid Test for Amines, what is the observable result for primary amines? Group of answer choices nitrogen gas bubbles form a soluble nitrite salt yellow oily layer of nitrosoaminearrow_forward3. a. Use the MS to propose at least two possible molecular formulas. For an unknown compound: 101. 27.0 29.0 41.0 50.0 52.0 55.0 57.0 100 57.5 58.0 58.5 62.0 63.0 64.0 65.0 74.0 40 75.0 76.0 20 20 40 60 80 100 120 140 160 180 200 220 m/z 99.5 68564810898409581251883040 115.0 116.0 77404799 17417M 117.0 12.9 118.0 33.5 119.0 36 133 0 1.2 157.0 2.1 159.0 16 169.0 219 170.0 17 171.0 21.6 172.0 17 181.0 1.3 183.0 197.0 100.0 198.0 200. 784 Relative Intensity 2 2 8 ō (ppm) 6 2arrow_forward
- Solve the structure and assign each of the following spectra (IR and C-NMR)arrow_forward1. For an unknown compound with a molecular formula of C8H100: a. What is the DU? (show your work) b. Solve the structure and assign each of the following spectra. 8 6 2 ō (ppm) 4 2 0 200 150 100 50 ō (ppm) LOD D 4000 3000 2000 1500 1000 500 HAVENUMBERI -11arrow_forward16. The proton NMR spectral information shown in this problem is for a compound with formula CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec- tral results, including DEPT-135 and DEPT-90 results, are tabulated: 7 J Normal Carbon DEPT-135 DEPT-90 19 ppm Positive No peak 122 Positive Positive cus и 124 Positive Positive 126 Positive Positive 128 No peak No peak 4° 129 Positive Positive 130 Positive Positive (144 No peak No peak 148 No peak No peak 150 Positive Positive してしarrow_forward
- 3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). + En CN CNarrow_forwardShow work..don't give Ai generated solution...arrow_forwardLabel the spectrum with spectroscopyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY