
(a)
Interpretation:
The preparation of the 1-bromo-3-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of
Activating groups – ortho/para directing groups. The
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(a)
Explanation of Solution
Given target compound,
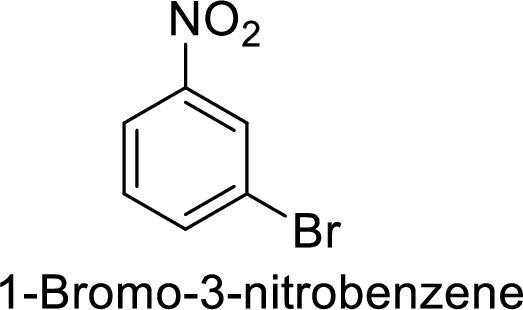
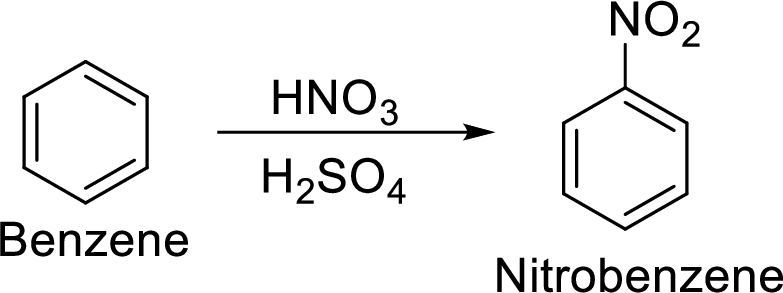
Step 1: Nitration of benzene ring by the reaction of benzene with nitrating mixture.
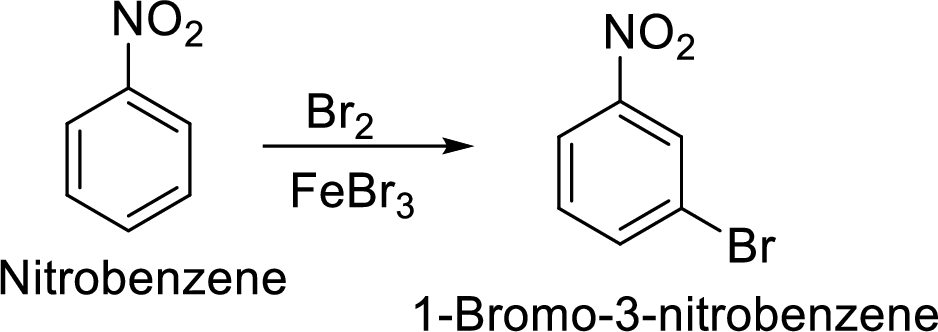
Step 2: Halogenation of nitrobenzene by the reaction of nitrobenzene with bromine in the presence of Lewis acid.
(b)
Interpretation:
The preparation of the 1-bromo-4-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(b)
Explanation of Solution
Given target compound,
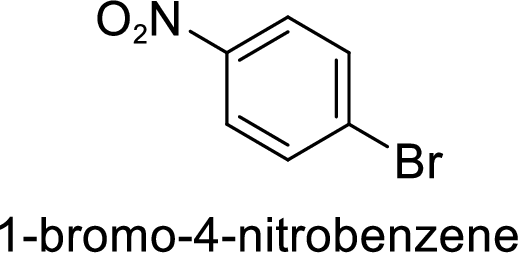
Step 1: Halogenation of benzene by the reaction of benzene with bromine in the presence of Lewis acid.
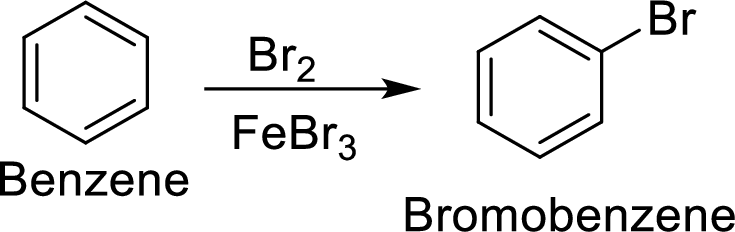
Step 2: Nitration of bromobenzene by the reaction of bromobenzene with nitrating mixture.
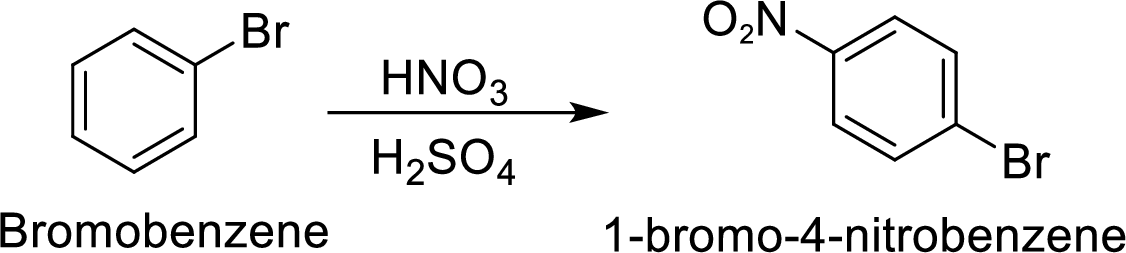
(c)
Interpretation:
The preparation of the 2,4-6-trinitrotoluene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(c)
Explanation of Solution
Given target compound,
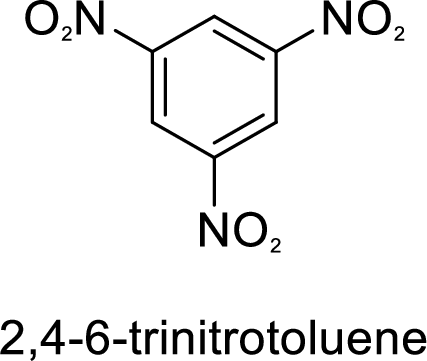
Methyl group in toluene is an activating and ortho-para directing group, so three times nitration of toluene gives the target compound.
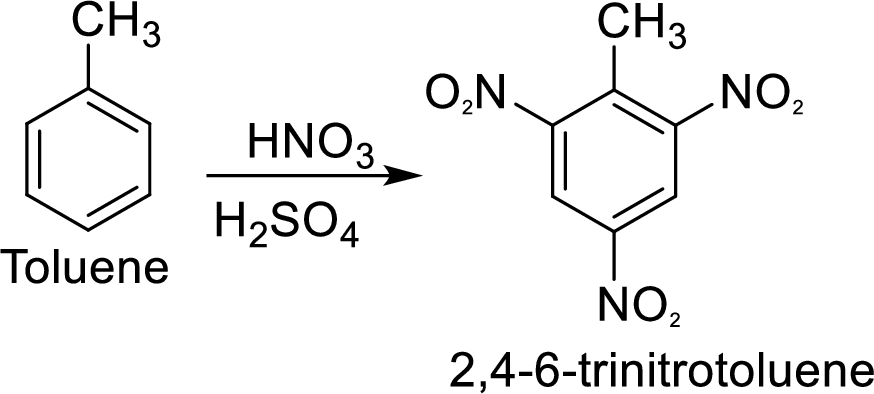
(d)
Interpretation:
The preparation of the m-chlorobenzoic acid from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(d)
Explanation of Solution
Given target compound,
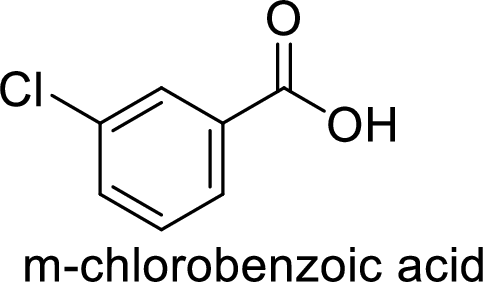
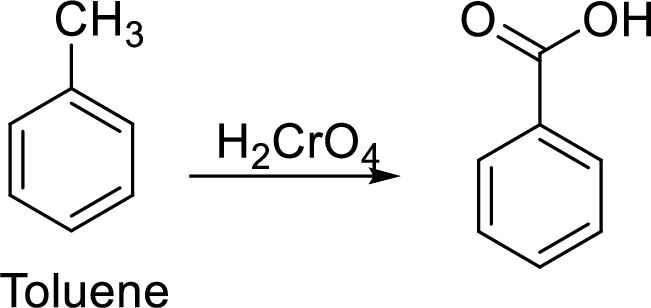
Step 1: Oxidation of toluene with chromic acid gives the benzoic acid.
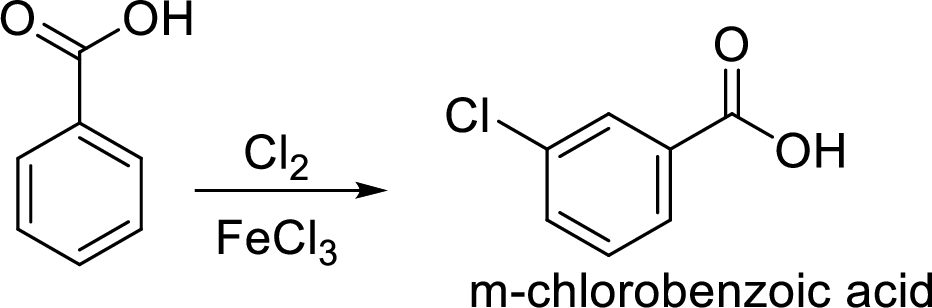
Step 2: Carboxyl group is a deactivating and meta directing group so the chlorination takes place at the meta position.
(e)
Interpretation:
The preparation of the p-chlorobenzoic acid from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(e)
Explanation of Solution
Given target compound,
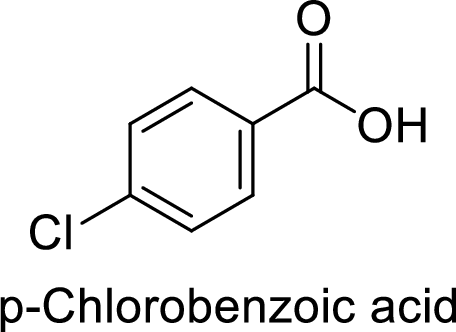
Step 1: Halogenation of toluene by the reaction of toluene with chlorine in the presence of Lewis acid.
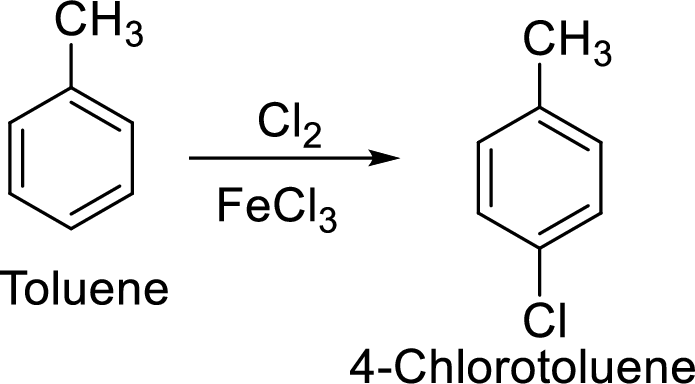
Step 2: Oxidation of 4-chlorotoluene by chromic acid gives the target compound.
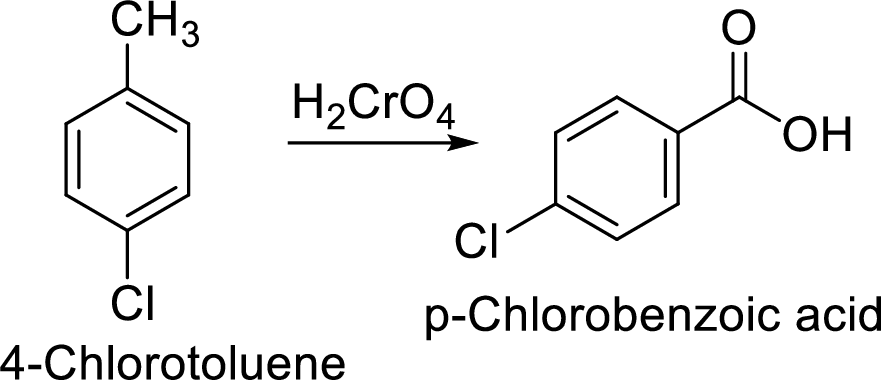
(f)
Interpretation:
The preparation of the p-dichlorobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(f)
Explanation of Solution
Given target compound,
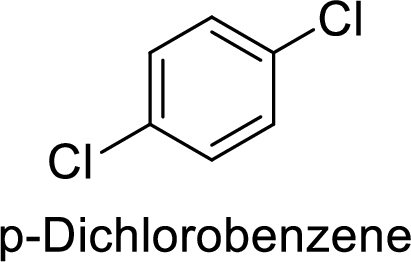
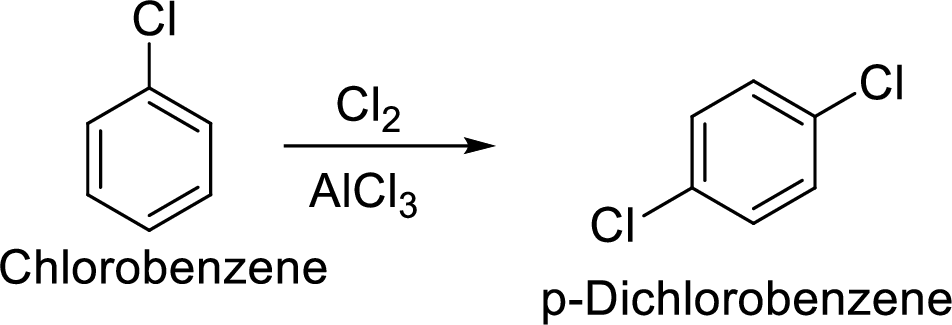
Step 1: Halogenation of benzene by the reaction of benzene with chlorine in the presence of Lewis acid.
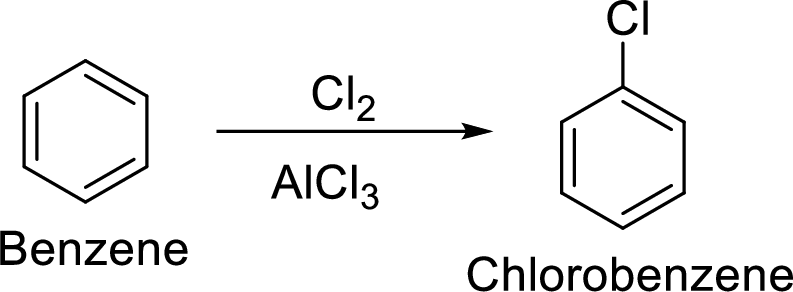
Step 2: Second halogenation of chlorobenzene by the reaction of chlorobenzene with chlorine in the presence of Lewis acid.
(g)
Interpretation:
The preparation of the 1-bromo-4-nitrobenzene from benzene or toluene or phenol has to be shown.
Concept Introduction:
Activating and deactivating groups:
The effect of substituents on the reaction rate of aromatic electrophilic substitution is given by activating or deactivating groups.
Activating groups – ortho/para directing groups. The rate of reaction is increased by an activating groups (electron donating groups) relative to hydrogen.
Deactivating groups – metadirecting groups. The rate of reaction is decreased by a deactivating groups (electron withdrawing groups) relative to hydrogen.
(g)
Explanation of Solution
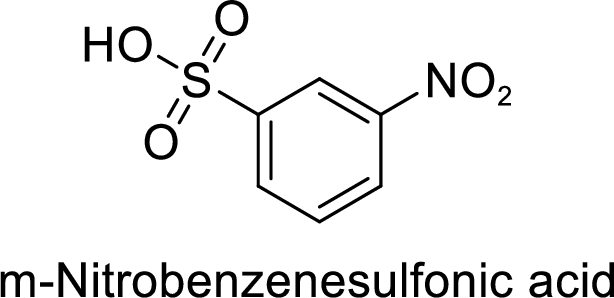
Given target compound,
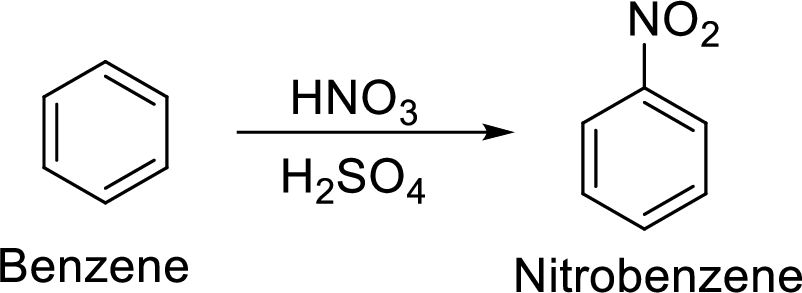
Step 1: Nitration of benzene by the reaction of benzene with nitrating mixture.
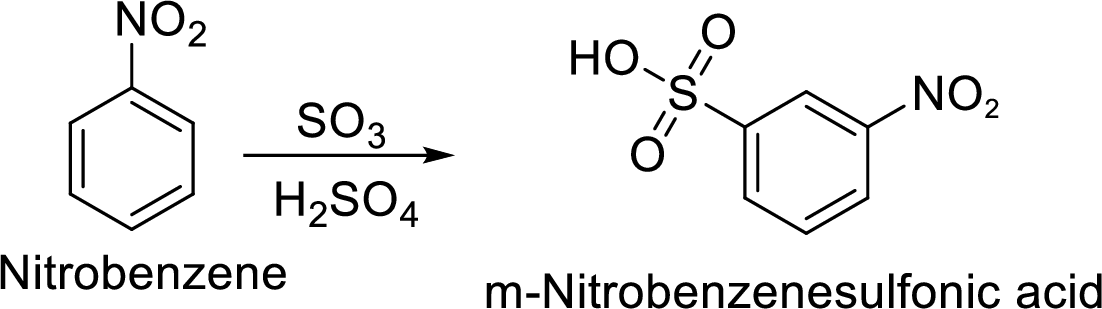
Step 2: Sulfonation of nitrobenzene by the reaction with
Want to see more full solutions like this?
Chapter 22 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

