
(a)
Interpretation: The acceptable names for the given compounds are to be predicted.
Concept introduction:
• The hydrocarbon is named after the longest carbon chain.
• The parent hydrocarbon containing ester group is named with suffix “ate”.
• The alkyl group bonded to oxygen through single bond forms the first part of the ester and alkyl group bonded to carbonyl group forms the second part.
Answer to Problem 22.38P
The acceptable name of the compound A is
Explanation of Solution
The given compound A is,
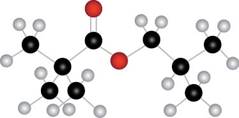
Figure 1
The red coloured ball has two bonds. So, this is the oxygen atom. The black coloured atoms have four single bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
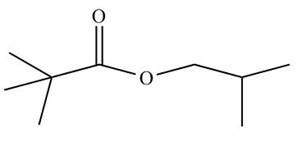
Figure 2
This compound contains ester group. The alkyl group attached to carbonyl group has longest chain of three carbon atoms with two methyl groups substituted at the second carbon atom. The alkyl group bonded to oxygen through single bond has longest chain of three carbon atoms with one methyl group substituted at the second carbon atom. The acceptable name of the compound is
The given compound B is,
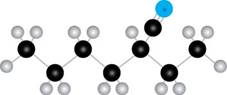
Figure 3
The blue coloured ball has three bonds. So, this is the nitrogen atom. The black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
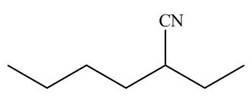
Figure 4
The given compound B contains longest chain of six carbon atoms with ethyl group substituted at the second carbon atom. The acceptable name of the compound is
The acceptable name of the compound A is
(b)
Interpretation: The products formed on treatment of A or B with given reagents: [1]
Concept introduction: The metal hydride reagents are good reducing agents such as
When the esters and nitriles are hydrolyzed to carboxylic acids in acidic medium and in basic medium carboxylate ions are formed.
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases.
Answer to Problem 22.38P
The reaction of compound A with given reagents are shown below.
[1]
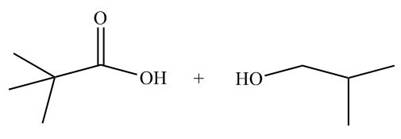
Figure 5
[2]
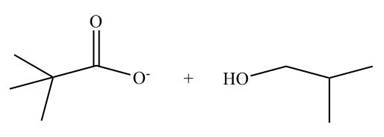
Figure 6
[3]
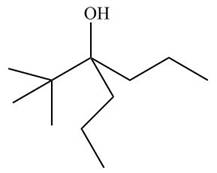
Figure 7
[4]
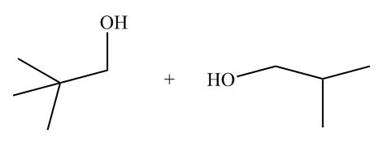
Figure 8
The reaction of compound B with given reagents are shown below.
[1]
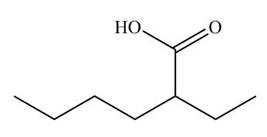
Figure 9
[2]
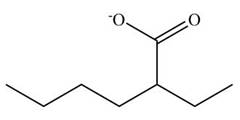
Figure 10
[3]
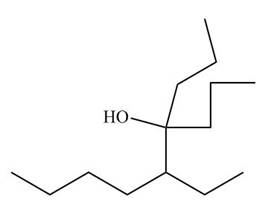
Figure 11
[4]
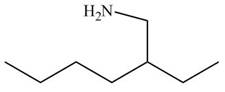
Figure 12
Explanation of Solution
The reaction of compound A with given reagents are shown below.
[1]
The ester group undergoes acidic hydrolysis to form alcohol and
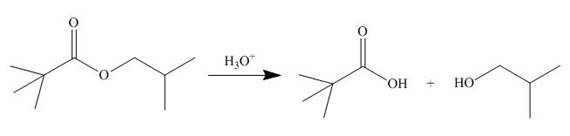
Figure 13
The products formed are
[2]
The ester group undergoes basic hydrolysis to form alcohol and carboxylic acid as shown below.
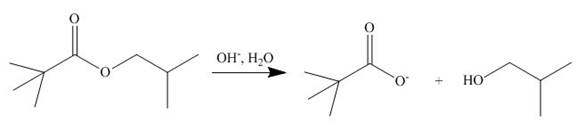
Figure 14
The products formed are
[3]
The given ester compound reacts with excess Grignard reagent followed by hydrolysis to form
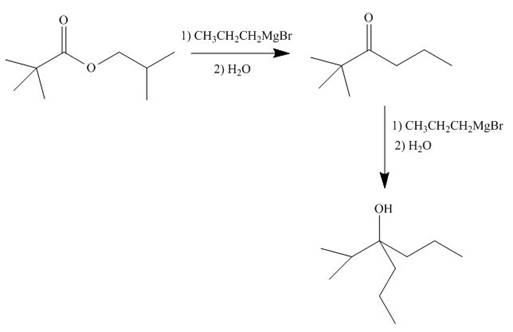
Figure 15
The product formed is form
[4]
In presence of lithium aluminium hydride, the ester group is reduced to two alcohols. The corresponding reaction is shown below.
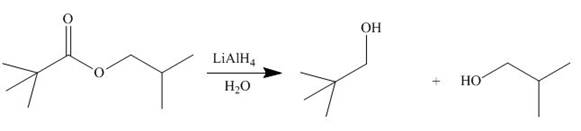
Figure 16
The products formed are
The reaction of compound B with given reagents are shown below.
[1]
The cyano group undergoes acidic hydrolysis to form carboxylic acid as shown below.
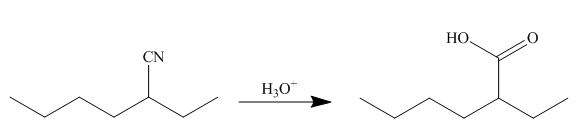
Figure 17
The products formed is
[2]
The cyano group undergoes basic hydrolysis to form carboxylate ion as shown below.
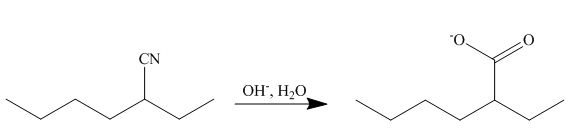
Figure 18
The product formed is
[3]
The nitrile compound reacts with Grignard reagent to form alcohol. The corresponding reaction is shown below.
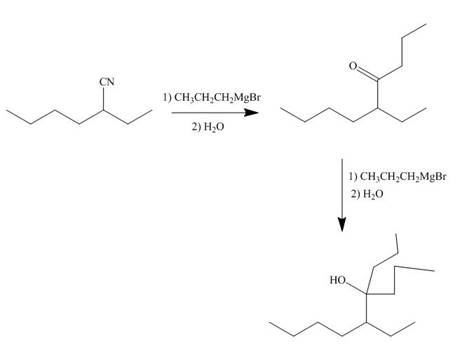
Figure 19
[4]
In the presence of lithium aluminium hydride, the ester group is reduced to two alcohols. The corresponding reaction is shown below.
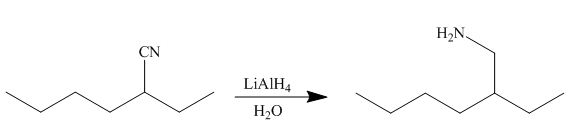
Figure 20
The product formed is
The products formed on treatment of A or B with given reagents: [1]
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
- For benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

