
At point A in a Carnot cycle, 2.34 mol of a monatomic ideal gas has a pressure of 1 4000 kPa, a volume of 10.0 L, and a temperature of 720 K. The gas expands isothermally to point B and then expands adiabatically to point C, where its volume is 24.0 L. An isothermal compression brings it to point D, where its volume is 15.0 L. An adiabatic process returns the gas to point A. (a) Determine all the unknown pressures, volumes, and temperatures as you f ill in the following table:
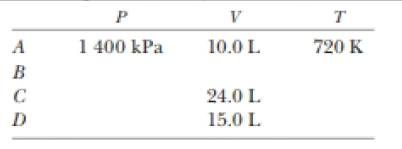
(b) Find the energy added by heat, the work done by the engine, and the change in internal energy for each of the steps A → B, B → C, C → D, and D → A (c) Calculate the efficiency Wnet/|Qk|. (d) Show that the efficiency is equal to 1 - TC/TA, the Carnot efficiency.
(a)
The unknown pressures, volumes and the temperature in the table.
Answer to Problem 22.32P
The values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation of adiabatic process
Here,
The value of
Substitute
Thus, the pressure of the gas at point
Write the ideal gas equation.
Here,
The value of gas constant is
Substitute
Thus, the temperature of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Write the equation of adiabatic process
Here,
Substitute
Thus, the volume of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Form a table and show the unknown value of pressures, volumes and temperatures.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
The energy added by heat, work done by the engine and the change in internal energy for each of the steps
Answer to Problem 22.32P
The values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
The process
Write the equation of change in temperature for process
Here,
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Here,
The value of
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
The process
Write the equation of change in temperature for process
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
Form a table and show the value of energy added by heat, work done by the engine and the change in internal energy.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
The value of efficiency
Answer to Problem 22.32P
The value of efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Calculate the net work done from the table is,
Write the equation for efficiency.
Here,
Substitute
The value of efficiency
Conclusion:
Therefore, the value of efficiency
(d)
To show: The efficiency is equal to the Carnot efficiency
Answer to Problem 22.32P
The efficiency is equal to the Carnot efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation for Carnot efficiency.
Here,
The value of
Substitute
Thus, the Carnot efficiency is
Write the equation for efficiency.
Substitute
The value of efficiency is
Conclusion:
Therefore, the efficiency is equal to the Carnot efficiency
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers
- Page 2 SECTION A Answer ALL questions in Section A [Expect to use one single-sided A4 page for each Section-A sub question.] Question A1 SPA6308 (2024) Consider Minkowski spacetime in Cartesian coordinates th = (t, x, y, z), such that ds² = dt² + dx² + dy² + dz². (a) Consider the vector with components V" = (1,-1,0,0). Determine V and V. V. (b) Consider now the coordinate system x' (u, v, y, z) such that u =t-x, v=t+x. [2 marks] Write down the line element, the metric, the Christoffel symbols and the Riemann curvature tensor in the new coordinates. [See the Appendix of this document.] [5 marks] (c) Determine V", that is, write the object in question A1.a in the coordinate system x'. Verify explicitly that V. V is invariant under the coordinate transformation. Question A2 [5 marks] Suppose that A, is a covector field, and consider the object Fv=AAμ. (a) Show explicitly that F is a tensor, that is, show that it transforms appropriately under a coordinate transformation. [5 marks] (b)…arrow_forwardHow does boiling point of water decreases as the altitude increases?arrow_forwardNo chatgpt pls will upvotearrow_forward
- 14 Z In figure, a closed surface with q=b= 0.4m/ C = 0.6m if the left edge of the closed surface at position X=a, if E is non-uniform and is given by € = (3 + 2x²) ŷ N/C, calculate the (3+2x²) net electric flux leaving the closed surface.arrow_forwardNo chatgpt pls will upvotearrow_forwardsuggest a reason ultrasound cleaning is better than cleaning by hand?arrow_forward
- Checkpoint 4 The figure shows four orientations of an electric di- pole in an external electric field. Rank the orienta- tions according to (a) the magnitude of the torque on the dipole and (b) the potential energy of the di- pole, greatest first. (1) (2) E (4)arrow_forwardWhat is integrated science. What is fractional distillation What is simple distillationarrow_forward19:39 · C Chegg 1 69% ✓ The compound beam is fixed at Ę and supported by rollers at A and B. There are pins at C and D. Take F=1700 lb. (Figure 1) Figure 800 lb ||-5- F 600 lb بتا D E C BO 10 ft 5 ft 4 ft-—— 6 ft — 5 ft- Solved Part A The compound beam is fixed at E and... Hình ảnh có thể có bản quyền. Tìm hiểu thêm Problem A-12 % Chia sẻ kip 800 lb Truy cập ) D Lưu of C 600 lb |-sa+ 10ft 5ft 4ft6ft D E 5 ft- Trying Cheaa Những kết quả này có hữu ích không? There are pins at C and D To F-1200 Egue!) Chegg Solved The compound b... Có Không ☑ ||| Chegg 10 וחarrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





