
Concept explainers
(a)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
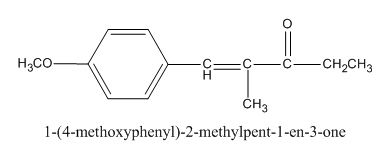
Figure 1
In the above preparation of the compound, the enolate is generated on the
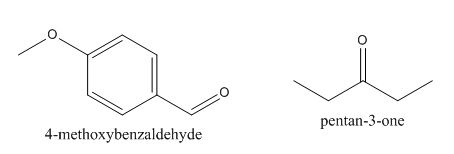
Figure 2
The given compound can be synthesized by aldol condensation of
(b)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are benzaldehyde and
Explanation of Solution
The given compound is shown below.
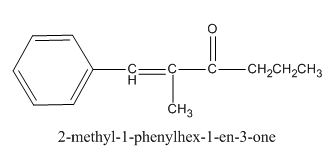
Figure 3
In the preparation of the above compound, the enolate will be generated on the
The structures of the starting materials which are used to produce the given compound are shown below.
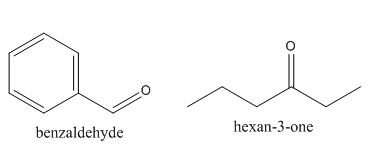
Figure 4
The given compound can be synthesized by aldol condensation of benzaldehyde and
(c)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation but not in a good in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
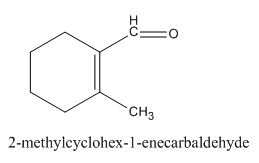
Figure 5
In the preparation of the above compound, intramolecular aldol condensation takes place. To form this compound enolate must be generated by aldehyde and it has to attack the carbonyl carbon of ketone which is not favorable condition. Favorable condition is that enolate should be generated on ketone which attacks the electrophilic carbon of the aldehyde. This is because the carbon of aldehyde is more acidic than the carbon of ketone. The structure of the starting material which is used to produce the given compound is shown below.
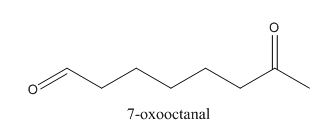
Figure 6
Therefore, the given compound cannot be synthesized by aldol condensation in a good yield.
This compound cannot be synthesized in a good yield by the aldol condensation.
(d)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
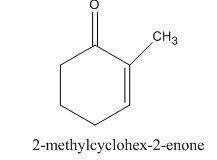
Figure 7
The given compound, is prepared by the intramolecular aldol condensation of
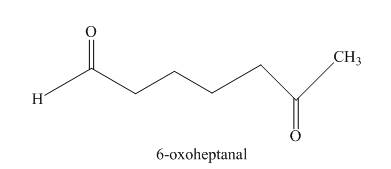
Figure 8
The given compound can be synthesized by intramolecular aldol condensation of
(e)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized from aldol condensation in a good yield. The starting materials are:
Explanation of Solution
The given compound is shown below.
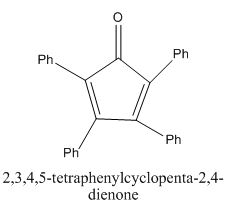
Figure 9
In the preparation of the given compound, the enolate will be generated on the
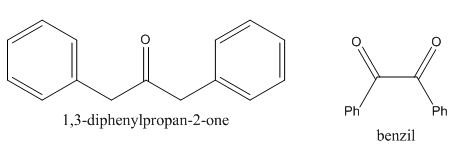
Figure 10
The given compound can be synthesized by aldol condensation of
(f)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
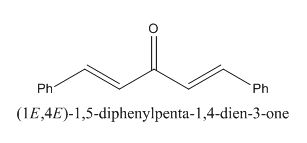
Figure 11
In the preparation of the compound, the enolate is generated on the
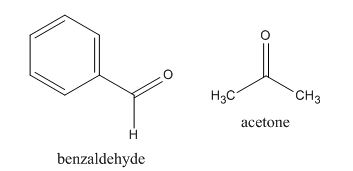
Figure 12
The given compound can be synthesized by aldol condensation of benzaldehyde and acetone.
(g)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation. The starting materials are trimethylacetone and acetaldehyde.
Explanation of Solution
The given compound is shown below.
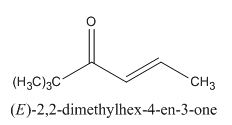
Figure 13
In the preparation of the given compound, the enolate will be generated on the
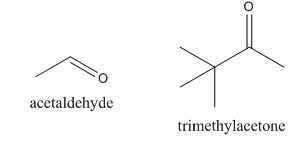
Figure 14
The given compound can be synthesized by aldol condensation by trimethylacetone and acetaldehyde in a good yield.
(h)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting material is
Explanation of Solution
The given compound is shown below.
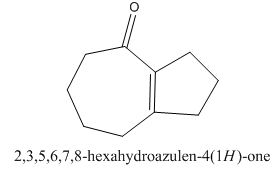
Figure 15
The enolate will be generated on the
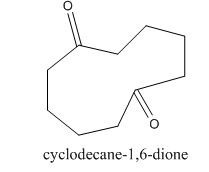
Figure 16
The given compound can be synthesized by aldol condensation of
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
- 21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forwardpls helparrow_forward27) Draw the energy level diagram and write the full and shorthand electron configuration for a neutral sulfur atom.arrow_forward
- Indicate whether these compounds are isomers, enantiomers, or tautomers. OCH OCH محمد ممدarrow_forward30) Substance A to E below are listed with several of their properties. The identities of the substances are identified in random order below: Iron, ethane, ethanol, sodium nitrate, graphite First classify each substance as either a polar covalent compound, non-polar covalent compound, ionic compound, metallic solid, or network solid. Write your predictions in the sixth coloumn of the chart, under "type of substance." Then, identify the identity of the substance in the last coloumn. Substance Melting Point Boiling Point Solubility in H₂O Electrical Conductivity Type of Substance Identity of Substance (°C) (°C) as: Solid, Liquids, Solution A -182 -88 Insoluble No/No/- B 1538 2862 Insoluble Yes/Yes/- C 308 380 Soluble Yes/Yes/Yes Ꭰ 3456 Insoluble No/-/- E -114 78 Soluble No/No/Noarrow_forwardpls helparrow_forward
- 28) Explain the process of galvanization. In your description, make sure to explain what metal is usually used for galvanization and why this metal used.arrow_forward29) Complete the following table Molecule H₂O NH3 Lewis Dot Diagram VSEPR Diagram Name of VSEPR Shapearrow_forward12) What is the best name to describe the shape of water molecule? a. Angular b. C. Tetrahedral Octahedral d. Trigonal pyramidal 13) Network solids are distinguished from metallic crystals in that: a. Network solids have charged ions, while metallic crystals do not. b. Network solids are composed of molecules, while metallic crystals only have one type of atom. C. Network solids are composed of non-metals. d. Network solids have much lower boiling points.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
