
Concept explainers
(a)
Interpretation:
Given compounds has to be arranged in their decreasing order of reactivity towards electrophilic
(a)
Explanation of Solution
Given compounds,
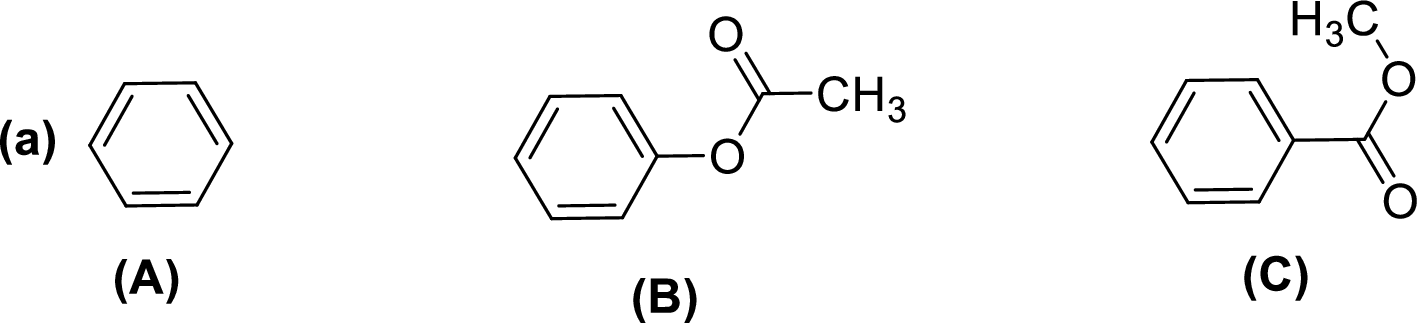
General trend of compounds reactivity in electrophilic aromatic substitution for a set of compounds having similar structure is given by,
So, electron donating substituents will increases the reactivity, while electron withdrawing substituents will decreases the reactivity.
Therefore, the decreasing order of reactivity towards electrophilic aromatic substitution of the given compounds are given below,
(b)
Interpretation:
Given compounds has to be arranged in their decreasing order of reactivity towards electrophilic aromatic substitution.
(b)
Explanation of Solution
Given compounds,
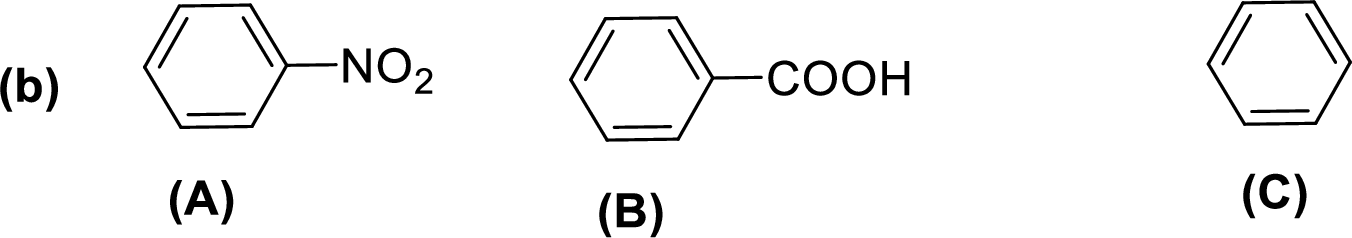
General trend of compounds reactivity in electrophilic aromatic substitution for a set of compounds having similar structure is given by,
So, electron donating substituents will increases the reactivity, while electron withdrawing substituents will decreases the reactivity.
Therefore, the decreasing order of reactivity towards electrophilic aromatic substitution of the given compounds are given below,
(c)
Interpretation:
Given compounds has to be arranged in their decreasing order of reactivity towards electrophilic aromatic substitution.
(c)
Explanation of Solution
Given compounds,
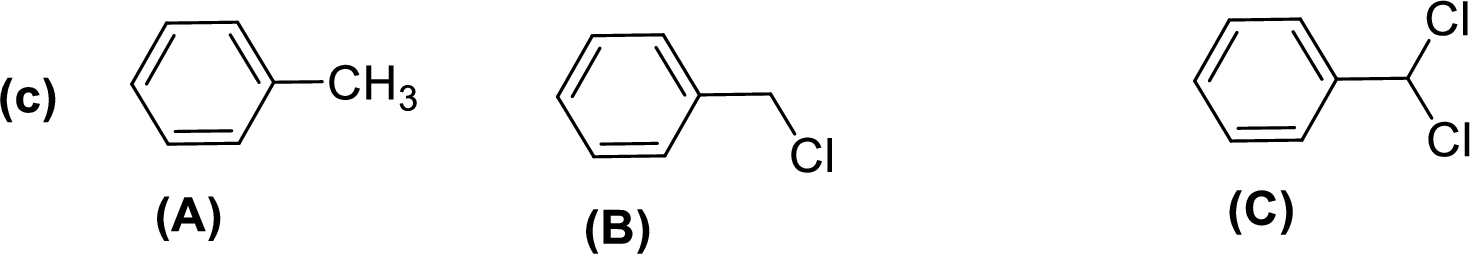
General trend of compounds reactivity in electrophilic aromatic substitution for a set of compounds having similar structure is given by,
So, electron donating substituents will increases the reactivity, while electron withdrawing substituents will decreases the reactivity.
Therefore, the decreasing order of reactivity towards electrophilic aromatic substitution of the given compounds are given below,
(d)
Interpretation:
Given compounds has to be arranged in their decreasing order of reactivity towards electrophilic aromatic substitution.
(d)
Explanation of Solution
Given compounds,
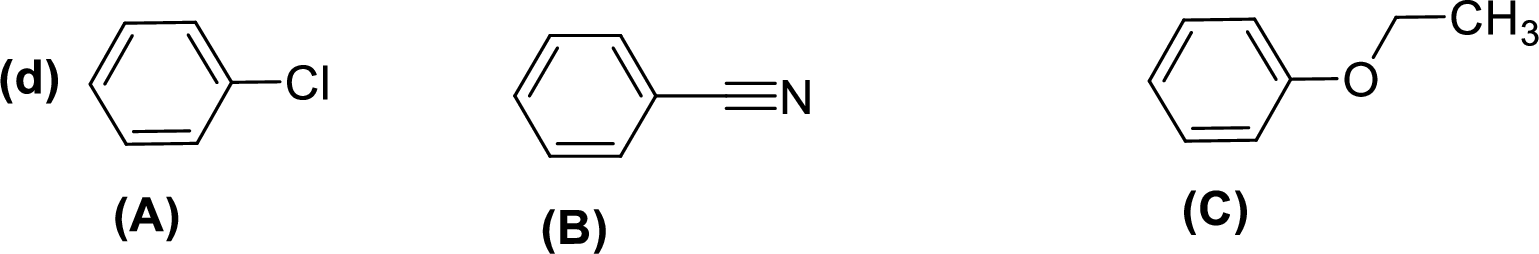
General trend of compounds reactivity in electrophilic aromatic substitution for a set of compounds having similar structure is given by,
So, electron donating substituents will increases the reactivity, while electron withdrawing substituents will decreases the reactivity.
Therefore, the decreasing order of reactivity towards electrophilic aromatic substitution of the given compounds are given below,
(e)
Interpretation:
Given compounds has to be arranged in their decreasing order of reactivity towards electrophilic aromatic substitution.
(e)
Explanation of Solution
Given compounds,
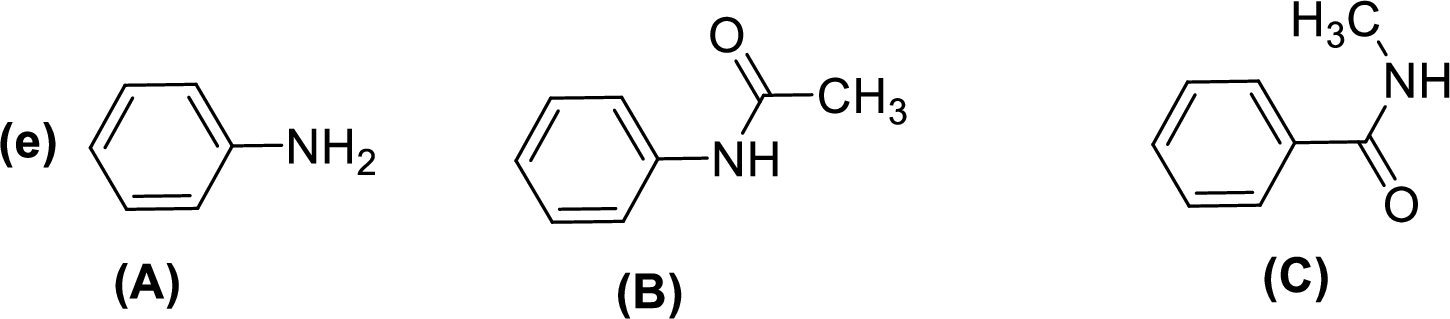
General trend of compounds reactivity in electrophilic aromatic substitution for a set of compounds having similar structure is given by,
So, electron donating substituents will increases the reactivity, while electron withdrawing substituents will decreases the reactivity.
Therefore, the decreasing order of reactivity towards electrophilic aromatic substitution of the given compounds are given below,
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forward
- Please answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forward
- Consider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forward
- What is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

