
Concept explainers
a. Use bond energies (see Table 3-3) to estimate ΔH for the reaction of two molecules of glycine to form a peptide linkage.
b. Would you predict ΔS to favor the formation of peptide linkages between two molecules of glycine?
c. Would you predict the formation of proteins to be a spontaneous process?
(a)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The
Answer to Problem 130AE
Answer
The
Explanation of Solution
Explanation
The enthalpy
The reaction between two molecules of glycine to form peptide linkage is,
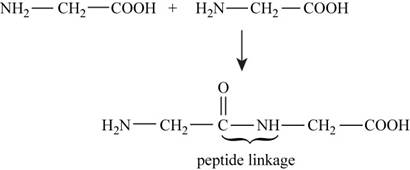
Figure 1
The enthalpy of the reaction is calculated by using the formula,
In the given reaction shown in Figure 1 bonds are broken and formed during peptide formation and energy is absorbed and released. The bond energies are shown in (Table 3-3) respectively.
Therefore, the energy required to break the bonds is calculated as,
Similarly, the energy released when the bonds are formed are calculated as,
Therefore, the total enthalpy for the reaction is calculated by using the formula,
Hence, the total enthalpy of formation for the reaction of two molecules of glycine to form a peptide linkage is positive. It is an endothermic reaction.
(b)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The entropy
Answer to Problem 130AE
Answer
The entropy
Explanation of Solution
Explanation
The entropy
The reaction between two molecules of glycine to form a peptide linkage is found to be an endothermic process because of the positive value of enthalpy. In the system an endothermic process absorbs heat from the surrounding and therefore, decreases the entropy of the surrounding because the molecular motion in the surrounding decreases. Therefore, for the given reaction
(c)
Interpretation: By using given bond energies the enthalpy
Concept introduction: When two or more amino acids are linked in a chain, a compound called peptide is formed in which the carboxyl group of each acid is joined to the amino group of the next forming a
To determine: The formation of proteins is a spontaneous process or not.
Answer to Problem 130AE
Answer
Formation of proteins is a non-spontaneous process.
Explanation of Solution
Explanation
The formation of proteins is a non-spontaneous process.
The formation of proteins occurs when large numbers of amino acids are joined through peptide linkage. The formation of peptide linkage is a non-spontaneous process as stated above due to the negative value of
Want to see more full solutions like this?
Chapter 22 Solutions
Bundle: Chemistry, 9th, Loose-Leaf + OWLv2 24-Months Printed Access Card
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





