
Concept explainers
Give IUPAC names for the following compounds:
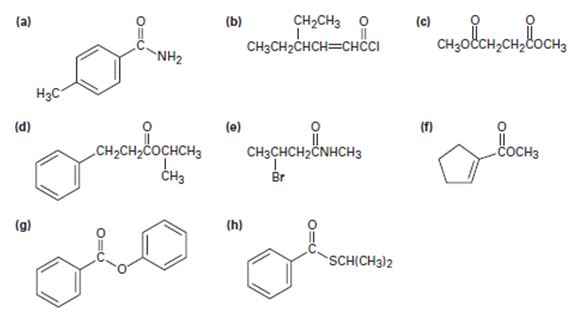
a)
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
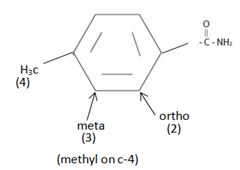
The IUPAC name of the given compound is p-methyl phenyl acet amide.
Explanation of Solution
The compound given is an unsubstituted amide that can be represented by the general formula 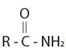 ; since the amide nitrogen in NH2 is not substituted by R1 an alkyl group.
; since the amide nitrogen in NH2 is not substituted by R1 an alkyl group.
The R alkyl group attached to carbonyl carbon is phenyl, which has a para-substituted methyl with the 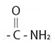 substituent. ie
substituent. ie
The IUPAC system of naming an amide unsubstituted follows this rule:
Replace the –oic acid or ic acid ending with amide and use the R alkyl group before this. Thus p-methyl-phenyl acetic acid to
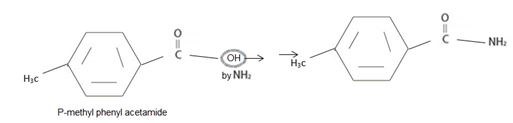
Thus the IUPAC name of the given compound is p-methyl phenyl acet amide.
b)
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
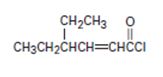
Thus the IUPAC name of the given compound is 4-ethyl-2-hexenoyl chloride.
Explanation of Solution
The given structure is consistent with 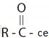 , an acyl chloride.
, an acyl chloride.
The IUPAC system names an acyl chloride by first identifying the alkyl component R by its IUPAC name. [the parent hydro carbon residue]. Obviously this given the name of the parent carboxylic acid itself. Follow this name by replacing the -ic acid ending with oyl and finally add the halide suffix.
For the given compound the parent hydrocarbon R is a six-carbon unsaturated hydrocarbon chain as.
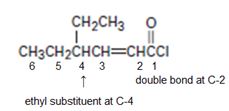
Thus this chain (longest chain) is named 4-ethyl-hexene-(2) [oic - acid] and the halide substituent chloride. We replace –oic acid by –oyl chloride. This leads to 4-ethyl-2 hexenoyl chloride or 4-ethyl-hexene-2-oyl chloride (from 4-ethyl-hexene-2-oic acid or 4 ethyl-2-hexenoic acid). The parent carboxylic acid.
Thus the IUPAC name of the given compound is 4-ethyl-2-hexenoyl chloride.
c)
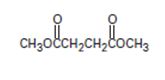
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
The IUPAC name of the given compound is thus dimethyl succinate.
Explanation of Solution
The given compound is
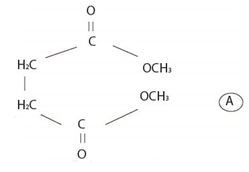
The structural formula A, of the given compound shows it to be the ester derivative of the dicarboxylic acid succinic acid is
The IUPAC system prescribes the following rule to name an ester derivative of a carboxylic acid thus:
First, identify the two alkyl group attached to each carboxylic oxygen (for two carboxylic acid groups in the given dicarboxylic acid). Then name the ester by starting with the name of their alkyl group (S) and adding the suffix–ate to replace the ending–ic acid.
Thus, combining these two rules, the given ester is named by IUPAC system as dimethyl succinate (from succinic acid) (for two CH3 groups) attached to carboxyl-groups)
The IUPAC name of the given compound is thus dimethyl succinate.
d)
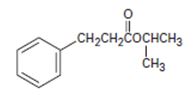
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
Thus, the IUPAC isopropyl-3 phenyl pro panoate name of the given compound is isopropyl–3 phenyl propanoate.
Explanation of Solution
The structural formula for the given compound is consistent with the general formula for an ester derivative of a mono carboxylic acid is 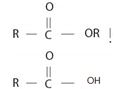 for the ester and for the parent acid the IUPAC system prescribes the following rules to name such an ester derivative is:
for the ester and for the parent acid the IUPAC system prescribes the following rules to name such an ester derivative is:
1) First, identify and name the alkyl group R1, attached to the carboxyl oxygen. For the given compound, it is isopropyl
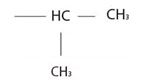
This corner in the prefix, first in the name of the parent carboxylic acid and also in the ester derivative.
2) Second, identify the R alkyl chain residue of the carboxylic acid. This for the given compound is the 3 carbon propanoic acid with a phenyl substituent at C-3 ie
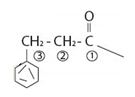
3) Combining these two rules, finally the use the suffix–ate to replace the–ic acid ending. This given.
Thus, the IUPAC isopropyl-3 phenyl pro panoate name of the given compound is isopropyl–3 phenyl propanoate.
e)
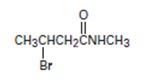
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
The IUPAC name of the given compound is N-methyl-4 bromo butanamide.
Explanation of Solution
The structural formula given for the compound is
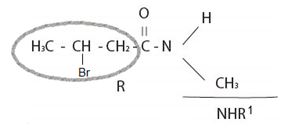
Consistent with the general formula for an amide derivative of a carbonylic acid is 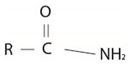 with the amide bring further alkyl, R1, substituted at the amide nitrogen ie NHR1 or
with the amide bring further alkyl, R1, substituted at the amide nitrogen ie NHR1 or 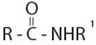 the IUPAC system prescribes the following ruler to name such substituted amides.
the IUPAC system prescribes the following ruler to name such substituted amides.
1) First, identify the N-alkyl substituent. In the given compound it is N-methyl-(mono substituted).
2) Next identify and name by the IUPAC system, the R, ie alkyl hydrocarbon chain component attached to the carbonyl carbon.
For the given compound, R is,
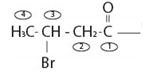
or remembering that carbonyl carbon is C-1, it is four carbon (longest hydro carbon chain) with a bromine at carbon-C-3. Thus, the name of this residue is 4-bromo butan–(derivative).
Finally, combining these two aspects, replace the–ic acid ending for the parent 4-bromobutanoic acid by –amide. This leads to N-methyl-4-bromo butanamide.
Thus the IUPAC name of the given compound is N-methyl-4 bromo butanamide.
f)
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
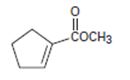
The IUPAC name for the given compound methyl-1-cyclopentene carboxylate.
Explanation of Solution
The IUPAC system prescribes the following rules to name such compounds is:
a) First, identify and name the R1, compound of the ester oxygen OR1. This, in the given compounds is methyl –OCH3.
b) Next identify the parent hydro carbon residue R attached the carbonyl carbon. For the given compound it is an unsaturated cyclopentene (5 carbon closed cyclic hydro carbon) with a double bond at C-1, which holds the carboxyl carbon. ie
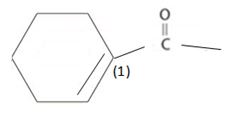
Thus, this residue R is 1-cyclopentene for the parent acid 1-cyclopentene carboxylic acid. Finally, combining these two rules, replace the –ic acid ending by –ate ie carboxylate. Thus, the name of the ester is methyl-1-cycopentene carboxylate.
Thus the IUPAC name for the given compound methyl-1-cyclopentene carboxylate.
g)
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
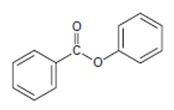
The IUPAC name of the given compound is phenyl-benzoate.
Explanation of Solution
The IUPAC system prescribes the following rules to name such esters is:
1) First identify and name the alkyl residue R1 of the ester oxygen. This, in the given compound is phenyl Ph or 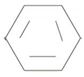 (unsubstituted)
(unsubstituted)
2) Second, identify and name by the IUPAC system the alkyl residue R attached to carbonyl carbon. This, in the given compound is
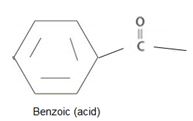
Finally combining these two rules, replace the –ic acid ending with the suffix –ate; the R1 alkyl name is the prefix. This leads to phenyl-benzoate.
Thus, the IUPAC name of the given compound is phenyl-benzoate.
h)
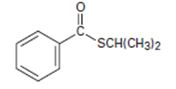
Interpretation:
To identify the given compound by introspecting its structural formula and name it by the IUPAC system.
Answer to Problem 45AP
Thus the IUPAC name for the given compound is isopropyl-phenyl since it is a thio ester carbothioate.
Explanation of Solution
The IUPAC system prescribes the following rules to name such thiol derivatives (sulphur) is:
1) First, identify and name by the IUPAC system, the alkyl residue, R1 attached to the –SR1 function. For the given compound this is the iso-propyl function ie
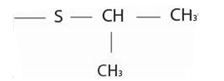
2) Next, identify and name the alkyl R residue attached to the carbonyl carbon. This is, for the given compound
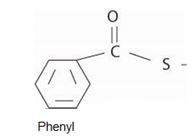
The parent carboxylic acid is thus phenyl carboxylic acid or
Finally, combining these two aspects, recall that the general ester of this carboxylic acid is the IUPAC name
Phenyl carboxylate for the this ester function, substitute the carboxylate ending by carbothioate. These the IUPAC name is, Isopropyl-phenyl carbothioate.
Thus the IUPAC name for the given compound is isopropyl-phenyl since it is a thio ester carbothioate.
Want to see more full solutions like this?
Chapter 21 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- What are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forward
- Can u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




