
ORGANIC CHEMISTRY W/ALEKS
6th Edition
ISBN: 9781264905430
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.3, Problem 6P
Which
a. 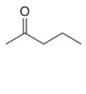 b.
b. 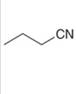 c.
c. 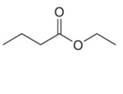 d.
d. 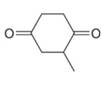
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the conjugate acid of C6H₂O¯ ?
A.
B.
C6H402-
C₂H4O
C. CH₂OH
ODCH₂OH
O E. C6H5OH3+
Which compound is considered as the conjugate base? *
D. OH-
B. C2H5NH+3
C. H2O
A. C2H5NH2
The atom in compound B that behaves only like a Bronsted–Lowry base is
the hydrogen bonded to oxygen.
the hydrogens bonded to nitrogen.
the oxygen atoms.
the nitrogen.
Chapter 21 Solutions
ORGANIC CHEMISTRY W/ALEKS
Ch. 21.2 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 21.2 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 21.3 - Prob. 5PCh. 21.3 - Problem 23.5 Which bonds in the following...Ch. 21.3 - Prob. 7PCh. 21.3 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.4 - Prob. 10PCh. 21.5 - Prob. 11PCh. 21.7 - Problem 23.11 Draw the products of each...
Ch. 21.7 - Problem 23.12 Draw the products of each reaction....Ch. 21.7 - Prob. 14PCh. 21.7 - Prob. 15PCh. 21.8 - Prob. 16PCh. 21.8 - Prob. 17PCh. 21.8 - Prob. 18PCh. 21.8 - Problem 23.18 How can pentan-2-one be converted...Ch. 21.8 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 21.9 - Problem 23.20 Which of the following compounds...Ch. 21.9 - Problem 23.21 Draw the products of each...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.9 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.10 - Prob. 27PCh. 21.10 - Prob. 28PCh. 21.10 - Prob. 29PCh. 21 - 23.29 Draw enol tautomer(s) for each compound....Ch. 21 - 22.30 The cis ketone A is isomerized to a trans...Ch. 21 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - 23.35 Rank the labeled protons in each compound in...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - 23.38 Acyclovir is an effective antiviral agent...Ch. 21 - 23.39 Explain why forms two different alkylation...Ch. 21 - Prob. 40PCh. 21 - 23.42 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - 23.45 Devise a synthesis of valproic acid , a...Ch. 21 - Prob. 57PCh. 21 - 23.57 Draw a stepwise mechanism showing how two...Ch. 21 - 23.58 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 65PCh. 21 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Several acids and their respective equilibrium constants are: Which is the strongest acid? Which is the weakest acid? Which acid has the weakest conjugate base? Which acid has the strongest conjugate base?arrow_forwardWhich hydrogen in each molecule is most acidic?arrow_forwardWhat is the correct answer and why?arrow_forward
- For each pair of molecules or ions, select the stronger base and write its Lewis structure. Q.CH3S- or CH3O-arrow_forwardDraw the products of each proton transfer reaction. Label the acid andbase in the starting materials, and the conjugate acid and base in theproducts.arrow_forwardWhich is the stronger acid?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY