
(a)
Interpretation:
Michael donor and Michael acceptor need to be identified for the given set of compounds.
Concept introduction:
When a active methylene is deprotonated to form a carbanion (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
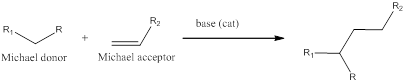
Please remember that 1,5-difunctionalization is the hallmark of Michael addition reaction.
To Find : To find the Michael donor and Michael acceptor for the given compound.
(b)
Interpretation:
Michael donor and Michael acceptor need to be identified for the given set of compounds.
Concept introduction:
When a active methylene is deprotonated to form a carbanion (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
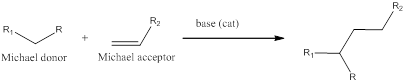
Please remember that 1,5-difunctionalization is the hallmark of Michael addition reaction.
To Find : To find the Michael donor and Michael acceptor for the given compound.
(c)
Interpretation:
Michael donor and Michael acceptor need to be identified for the given set of compounds.
Concept introduction:
When a active methylene is deprotonated to form a carbanion (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
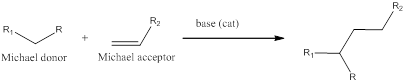
Please remember that 1,5-difunctionalization is the hallmark of Michael addition reaction.
To Find : To find the Michael donor and Michael acceptor for the given compound.
(d)
Interpretation:
Michael donor and Michael acceptor need to be identified for the given set of compounds.
Concept introduction:
When a active methylene is deprotonated to form a carbanion (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
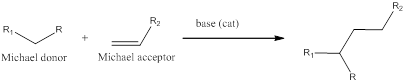
To Find : To find the Michael donor and Michael acceptor for the given compound.
(e)
Interpretation:
Michael donor and Michael acceptor need to be identified for the given set of compounds.
Concept introduction:
When a active methylene is deprotonated to form a carbanion (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
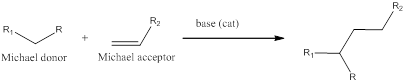
Please remember that 1,5-difunctionalization is the hallmark of Michael addition reaction.
To Find : To find the Michael donor and Michael acceptor for the given compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





