
(a)
Interpretation: The boiling point and freezing point of the given solution of glucose in ethanol should be determined.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
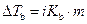
Where,
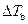 is the elevation in boiling point.
is the elevation in boiling point.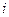 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.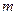 is the molality of the solution.
is the molality of the solution.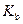 is the molal boiling point elevation constant
is the molal boiling point elevation constant 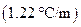 refer table 13.3)
refer table 13.3)
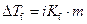
Where,
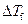 is the depression in freezing point.
is the depression in freezing point.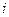 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.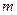 is the molality of the solution.
is the molality of the solution.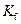 is the molal freezing point depression constant
is the molal freezing point depression constant 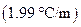 refer table13.3)
refer table13.3)
(b)
Interpretation: The boiling point and freezing point of the given solution of decane in chloroform should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
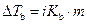
Where,
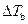 is the elevation in boiling point.
is the elevation in boiling point.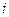 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.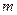 is the molality of the solution.
is the molality of the solution.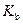 is the molal boiling point elevation constant
is the molal boiling point elevation constant 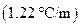 refer table 13.3)
refer table 13.3)
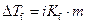
Where,
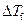 is the depression in freezing point.
is the depression in freezing point.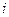 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.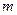 is the molality of the solution.
is the molality of the solution.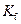 is the molal freezing point depression constant
is the molal freezing point depression constant 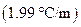 refer table13.3)
refer table13.3)
(c)
Interpretation: The boiling point and freezing point of the given solution of 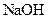 in water should be calculated.
in water should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
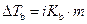
Where,
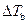 is the elevation in boiling point.
is the elevation in boiling point.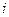 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.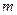 is the molality of the solution.
is the molality of the solution.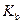 is the molal boiling point elevation constant
is the molal boiling point elevation constant 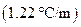 refer table 13.3)
refer table 13.3)
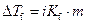
Where,
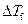 is the depression in freezing point.
is the depression in freezing point.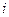 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.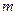 is the molality of the solution.
is the molality of the solution.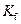 is the molal freezing point depression constant
is the molal freezing point depression constant 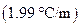 refer table13.3)
refer table13.3)
(d)
Interpretation: The boiling point and freezing point of the given solution of ethylene glycol and 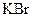 in water should be calculated.
in water should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
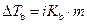
Where,
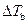 is the elevation in boiling point.
is the elevation in boiling point.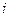 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.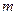 is the molality of the solution.
is the molality of the solution.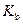 is the molal boiling point elevation constant
is the molal boiling point elevation constant 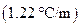 refer table 13.3)
refer table 13.3)
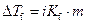
Where,
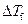 is the depression in freezing point.
is the depression in freezing point.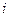 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.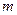 is the molality of the solution.
is the molality of the solution.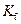 is the molal freezing point depression constant
is the molal freezing point depression constant 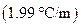 refer table13.3)
refer table13.3)
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Chemistry the Central Science 13th Edition Custom for Lamar University
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





