
Concept explainers
The reaction between reactant A (blue spheres) and reactant B (red spheres) is shown in the
following diagram
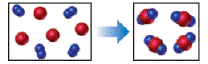
Based on this diagram, which equation best describes the reaction? [Section 3.1D
b.
c.
d.
To determine: The equation that best describes the reaction shown in the given diagram.
Answer to Problem 1E
Solution: The equation (a)
Explanation of Solution
Given
The reaction diagram,
Figure 1 The blue spheres represent the reactant
The red spheres represent the reactant
As depicted in the given diagram, A or blue spheres are present as diatomic molecules
4 molecules of A,
The reaction will be:
Taking coefficients common,
The equation that best describes the depicted chemical reaction is (a)
Want to see more full solutions like this?
Chapter 1 Solutions
Chemistry the Central Science 13th Edition Custom for Lamar University
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Biology: Life on Earth (11th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
The Cosmic Perspective (8th Edition)
- Problem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forwardGas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward
- 10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forwardProvide steps to name and label for understanding.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





