
(a)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(a)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is phenyl formate.
The molecular formula of phenyl formate is
The structure of phenyl formate is given as,
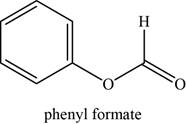
Figure 1
(b)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(b)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclohexyl benzoate.
The molecular formula of cyclohexyl benzoate is
The structure of cyclohexyl benzoate is given as,
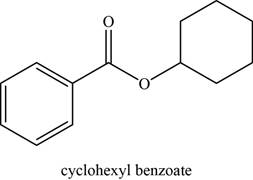
Figure 2
(c)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(c)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclopentyl phenylacetate.
The molecular formula of cyclopentyl phenylacetate is
The structure of cyclopentyl phenylacetate is given as,
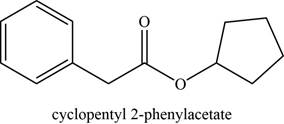
Figure 3
(d)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(d)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is N-butylacetamide.
The molecular formula of N-butylacetamide is
The structure of N-butylacetamide is given as,
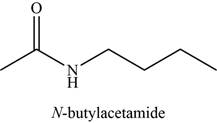
Figure 4
(e)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(e)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is N,N-dimethylformamide.
The molecular formula of N,N-dimethylformamide is
The structure of N,N-dimethylformamide is given as,
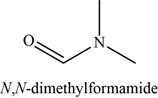
Figure 5
(f)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(f)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is benzoic propionic anhydride.
The molecular formula of benzoic propionic anhydride is
The structure of benzoic propionic anhydride is given as,
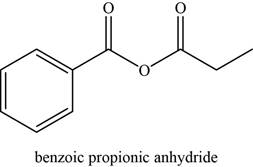
Figure 6
(g)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(g)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is benzamide.
The molecular formula of benzamide is
The structure of benzamide is given as,
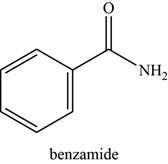
Figure 7
(h)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(h)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
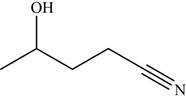
Figure 8
(i)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(i)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
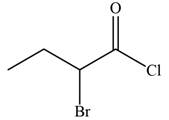
Figure 9
(j)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(j)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
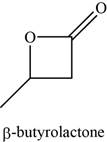
Figure 10
(k)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(k)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is phenyl isocyanate.
The molecular formula of phenyl isocyanate is
The structure of phenyl isocyanate is given as,
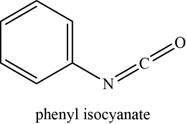
Figure 11
(l)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(l)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is cyclobutyl ethyl carbonate.
The molecular formula of cyclobutyl ethyl carbonate is
The structure of cyclobutyl ethyl carbonate is given as,
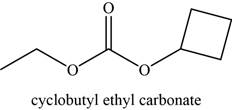
Figure 12
(m)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(m)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is
The molecular formula of
The structure of
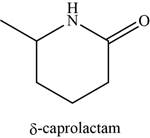
Figure 13
(n)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(n)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is trichloroacetic anhydride.
The molecular formula of trichloroacetic anhydride is
The structure of trichloroacetic anhydride is given as,
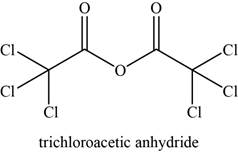
Figure 14
(o)
Interpretation:
The structure to correspond with the given common and systematic names is to be drawn.
Concept introduction:
Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because common names do not follow any rule, whereas IUPAC names follow specific rules. The common name does not include any suffix, prefix, and numbers.
(o)
Answer to Problem 21.42SP
The structure to correspond with the given common and systematic names is given below.
Explanation of Solution
The given compound is Ethyl-N-methyl carbamate.
The molecular formula of Ethyl-N-methyl carbamate is
The structure of Ethyl-N-methyl carbamate is given as,
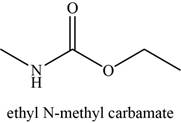
Figure 15
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY II LAB MANUAL>CUSTOM<
- One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forward
- Benzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forward
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

