
(a)
Interpretation:
The complete, detailed mechanism of a given compound synthesized from an acid chloride and water is to be drawn.
Concept introduction:
Answer to Problem 21.40P
The complete mechanism is
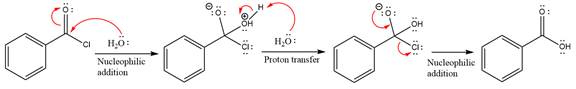
Explanation of Solution
The given molecule is
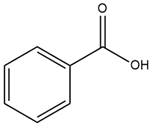
The carboxylic acid formation requires water as a nucleophile in the nucleophilic addition step. In this reaction,
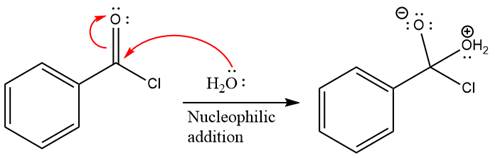
In the first step, water acts as a nucleophile and adds to the carbonyl carbon of the acid chloride to produce a protonated intermediate ion. The
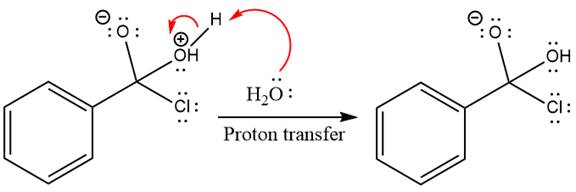
The protonated water is then deprotonated by a second molecule of the water.
Finally, one lone pair on the negatively charged oxygen will move back to reform the carbonyl group, eliminating the chloride and forming the product.
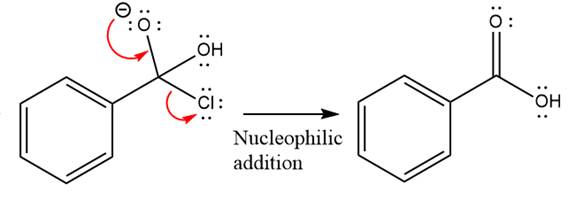
Thus, the complete mechanism can be drawn as
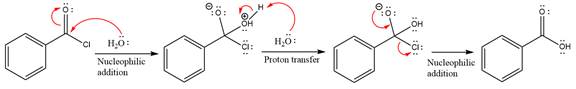
The product and mechanism of the given reaction were determined based on nucleophilic addition-elimination mechanism.
(b)
Interpretation:
The complete, detailed mechanism of a given compound synthesized from an acid chloride and water is to be drawn.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly.
Answer to Problem 21.40P
The complete mechanism is
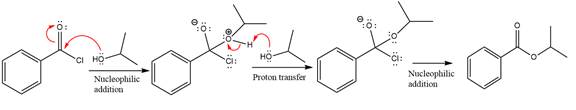
Explanation of Solution
The given molecule is
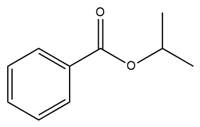
The ester formation requires isopropanol as a nucleophile in the nucleophilic addition step. In this reaction,
In the first step, isopropanol acts as a nucleophile and adds to the carbonyl carbon of the acid chloride to produce a protonated intermediate ion. The
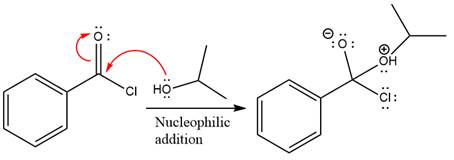
The protonated alcohol is then deprotonated by a second molecule of the isopropanol.
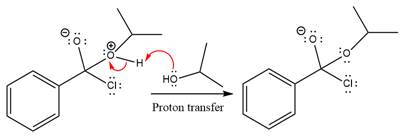
Finally, one lone pair on the negatively charged oxygen will move back to reform the carbonyl group, eliminating the chloride and forming the product.
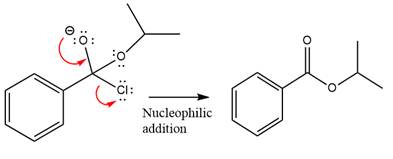
Thus, the complete mechanism can be drawn as

The product and mechanism of the given reaction were determined based on nucleophilic addition-elimination mechanism.
(c)
Interpretation:
The complete, detailed mechanism of a given compound synthesized from an acid chloride and water is to be drawn.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly.
Answer to Problem 21.40P
The complete mechanism is
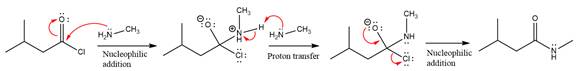
Explanation of Solution
The given molecule is
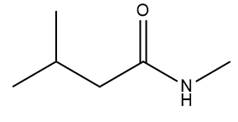
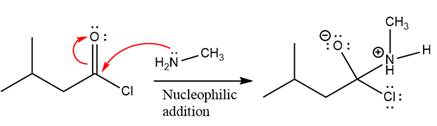
The amide formation requires
In the first step, amine acts as a nucleophile and adds to the carbonyl carbon of the acid chloride to produce a protonated intermediate ion. The
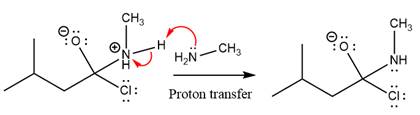
The protonated amine is then deprotonated by a second molecule of the amine.
Finally, one lone pair on the negatively charged oxygen will move back to reform the carbonyl group, eliminating the chloride and forming the product.
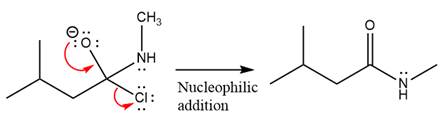
Thus, the complete mechanism can be drawn as
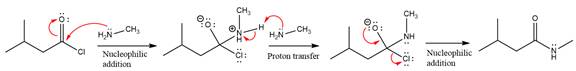
The product and mechanism of the given reaction were determined on the basis of nucleophilic addition-elimination mechanism.
(d)
Interpretation:
The complete, detailed mechanism of given compound synthesized from an acid chloride and water is to be drawn.
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s), particularly when the nucleophile being added in the first step is not a strong nucleophile. The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly.
Answer to Problem 21.40P
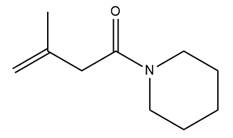
The complete mechanism is
Explanation of Solution
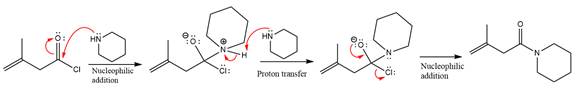
The given molecule is
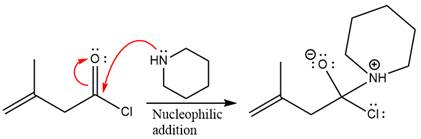
The amide formation requires amine as a nucleophile in the nucleophilic addition step. In this reaction,
In the first step, pyridine acts as a nucleophile and adds to the carbonyl carbon of the acid chloride to produce a protonated intermediate ion. The
The protonated pyridine is then deprotonated by a second molecule of the amine.
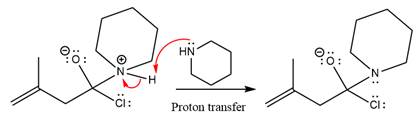
Finally, one lone pair on the negatively charged oxygen will move back to reform the carbonyl group, eliminating the chloride and forming the product.
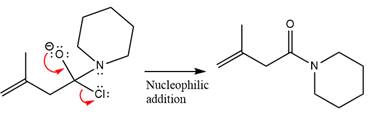
Thus, the complete mechanism can be drawn as
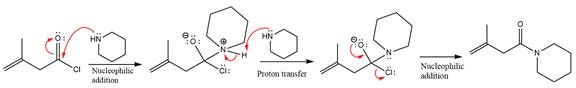
The product and mechanism of the given reaction were determined based on nucleophilic addition-elimination mechanism.
Want to see more full solutions like this?
Chapter 21 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





