
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN: 9781305116399
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 21.2QQ
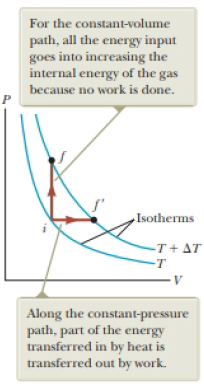
(i) How does the internal energy of an ideal gas change as it follows path i → f in Figure 20.4? (a) Eint increases. (b) Eint decreases. (c) Eint stays the same. (d) There is not enough information to determine how Eint changes. (ii) From the same choices, how does the internal energy of an ideal gas change as it follows path f → f′ along the isotherm labeled T + ΔT in Figure 20.4?
Figure 20.4 Energy is transferred by heat to an ideal gas in two ways.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
If the metal sphere on the Van de Graff has a charge of 0.14 Coulombs and the person has a mass of 62 kg, how much excess charge would the person need in order to levitate at a distance 25 cm from the center of the charged metal sphere? Assume you can treat both the person and the metal sphere as point charges a distance 25 cm from each other
If the metal sphere on the Van de Graff has a charge of 0.14 Coulombs and the person has a mass of 62 kg, how much excess charge would the person need in order to levitate at a distance 25 cm from the center of the charged metal sphere? Assume you can treat both the person and the metal sphere as point charges a distance 25 cm from each other (so that you can use Coulomb's Law to calculate the electrical force).
Using Coulomb's Law, calculate the magnitude of the electrical force between two protons located 1 meter apart from each other. (Give your answer as the number of Newtons but as usual you only need to include the number, not the unit label.)
Chapter 21 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
Ch. 21 - Two containers hold an ideal gas at the same...Ch. 21 - (i) How does the internal energy of an ideal gas...Ch. 21 - Prob. 21.3QQCh. 21 - Prob. 21.4QQCh. 21 - Cylinder A contains oxygen (O2) gas, and cylinder...Ch. 21 - An ideal gas is maintained at constant pressure....Ch. 21 - Prob. 21.3OQCh. 21 - A helium-filled latex balloon initially at room...Ch. 21 - Prob. 21.5OQCh. 21 - Prob. 21.6OQ
Ch. 21 - A sample of gas with a thermometer immersed in the...Ch. 21 - Prob. 21.8OQCh. 21 - Which of the assumptions below is not made in the...Ch. 21 - Hot air rises, so why does it generally become...Ch. 21 - Prob. 21.2CQCh. 21 - When alcohol is rubbed on your body, it lowers...Ch. 21 - What happens to a helium-filled latex balloon...Ch. 21 - Which is denser, dry air or air saturated with...Ch. 21 - One container is filled with helium gas and...Ch. 21 - Daltons law of partial pressures states that the...Ch. 21 - (a) How many atoms of helium gas fill a spherical...Ch. 21 - A cylinder contains a mixture of helium and argon...Ch. 21 - Prob. 21.3PCh. 21 - In an ultrahigh vacuum system (with typical...Ch. 21 - A spherical balloon of volume 4.00 103 cm3...Ch. 21 - A spherical balloon of volume V contains helium at...Ch. 21 - A 2.00-mol sample of oxygen gas is confined to a...Ch. 21 - Oxygen, modeled as an ideal gas, is in a container...Ch. 21 - Prob. 21.9PCh. 21 - The rms speed of an oxygen molecule (O2) in a...Ch. 21 - A 5.00-L vessel contains nitrogen gas at 27.0C and...Ch. 21 - A 7.00-L vessel contains 3.50 moles of gas at a...Ch. 21 - In a period of 1.00 s, 5.00 1023 nitrogen...Ch. 21 - In a constant-volume process, 209 J of energy is...Ch. 21 - A sample of a diatomic ideal gas has pressure P...Ch. 21 - Review. A house has well-insulated walls. It...Ch. 21 - A 1.00-mol sample of hydrogen gas is healed at...Ch. 21 - A vertical cylinder with a heavy piston contains...Ch. 21 - Calculate the change in internal energy of 3.00...Ch. 21 - A 1.00-L insulated bottle is full of tea at 90.0C....Ch. 21 - Review. This problem is a continuation of Problem...Ch. 21 - A certain molecule has f degrees of freedom. Show...Ch. 21 - In a crude model (Fig. P21.23) of a rotating...Ch. 21 - Why is the following situation impossible? A team...Ch. 21 - Prob. 21.25PCh. 21 - A 2.00-mol sample of a diatomic ideal gas expands...Ch. 21 - During the compression stroke of a certain...Ch. 21 - How much work is required to compress 5.00 mol of...Ch. 21 - Air in a thundercloud expands as it rises. If its...Ch. 21 - Why is the following situation impossible? A new...Ch. 21 - During the power stroke in a four-stroke...Ch. 21 - Air (a diatomic ideal gas) at 27.0C and...Ch. 21 - A 4.00-L sample of a diatomic ideal gas with...Ch. 21 - Prob. 21.34PCh. 21 - Prob. 21.35PCh. 21 - Fifteen identical particles have various speeds:...Ch. 21 - Prob. 21.37PCh. 21 - Prob. 21.38PCh. 21 - Prob. 21.39PCh. 21 - Consider a container of nitrogen gas molecules at...Ch. 21 - Prob. 21.41PCh. 21 - Prob. 21.42PCh. 21 - The law of atmospheres states that the number...Ch. 21 - Prob. 21.44APCh. 21 - Prob. 21.45APCh. 21 - The dimensions of a classroom are 4.20 m 3.00 m ...Ch. 21 - The Earths atmosphere consists primarily of oxygen...Ch. 21 - Prob. 21.48APCh. 21 - An air rifle shoots a lead pellet by allowing high...Ch. 21 - Prob. 21.50APCh. 21 - A certain ideal gas has a molar specific heat of...Ch. 21 - Prob. 21.52APCh. 21 - Review. Oxygen at pressures much greater than 1...Ch. 21 - Prob. 21.54APCh. 21 - Model air as a diatomic ideal gas with M = 28.9...Ch. 21 - Review. As a sound wave passes through a gas, the...Ch. 21 - Prob. 21.57APCh. 21 - In a cylinder, a sample of an ideal gas with...Ch. 21 - As a 1.00-mol sample of a monatomic ideal gas...Ch. 21 - A sample consists of an amount n in moles of a...Ch. 21 - Prob. 21.61APCh. 21 - A vessel contains 1.00 104 oxygen molecules at...Ch. 21 - A pitcher throws a 0.142-kg baseball at 47.2 m/s....Ch. 21 - The latent heat of vaporization for water at room...Ch. 21 - A sample of a monatomic ideal gas occupies 5.00 L...Ch. 21 - Prob. 21.66APCh. 21 - Prob. 21.67APCh. 21 - Prob. 21.68APCh. 21 - Prob. 21.69APCh. 21 - On the PV diagram for an ideal gas, one isothermal...Ch. 21 - Prob. 21.71APCh. 21 - Review, (a) H it has enough kinetic energy, a...Ch. 21 - Prob. 21.73APCh. 21 - Prob. 21.74CPCh. 21 - A cylinder is closed at both ends and has...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Part A You want to get an idea of the magnitude of magnetic fields produced by overhead power lines. You estimate that a transmission wire is about 12 m above the ground. The local power company tells you that the line operates at 12 kV and provide a maximum of 60 MW to the local area. Estimate the maximum magnetic field you might experience walking under such a power line, and compare to the Earth's field. [For an ac current, values are rms, and the magnetic field will be changing.] Express your answer using two significant figures. ΟΤΕ ΑΣΦ VAΣ Bmax= Submit Request Answer Part B Compare to the Earth's field of 5.0 x 10-5 T. Express your answer using two significant figures. Ο ΑΣΦ B BEarth ? ? Tarrow_forwardHo propel 9-kN t. Boat 27. An elevator accelerates downward at 2.4 m/s². What force does the elevator's floor exert on a 52-kg passenger?arrow_forward16. 17 A CUIN Starting from rest and undergoing constant acceleration, a 940-kg racing car covers 400 m in 4.95 s. Find the force on the car.arrow_forward
- ----- vertical diste Section 4.6 Newton's Third Law 31. What upward gravitational force does a 5600-kg elephant exert on Earth?arrow_forward64. Two springs have the same unstretched length but different spring constants, k₁ and k₂. (a) If they're connected side by side and stretched a distance x, as shown in Fig. 4.24a, show that the force exerted by the combination is (k₁ + k₂)x. (b) If they're con- nected end to end (Fig. 4.24b) and the combination is stretched a distance x, show that they exert a force k₁k2x/(k₁ + k₂). www (a) FIGURE 4.24 Problem 65 www (b)arrow_forward65. Although we usually write Newton's second law for one-dimensional motion in the form F =ma, which holds when mass is constant, d(mv) a more fundamental version is F = . Consider an object dt whose mass is changing, and use the product rule for derivatives to show that Newton's law then takes the form F dm = ma + v dtarrow_forward
- If a proton is located on the x-axis in some coordinate system at x0 = -3.2 x 10-5 meters, what is the x-component of the Electric Field due to this proton at a position x = +3.2 x 10-5 meters and on the x axis as the y-axis is 0 giving a number of Newtons/Coulomb?arrow_forwardConsider a single square loop of wire of area A carrying a current I in a uniform magnetic field of strength B. The field is pointing directly up the page in the plane of the page. The loop is oriented so that the plane of the loop is perpendicular to the plane of the page (this means that the normal vector for the loop is always in the plane of the page!). In the illustrations below the magnetic field is shown in red and the current through the current loop is shown in blue. The loop starts out in orientation (i) and rotates clockwise, through orientations (ii) through (viii) before returning to (i). (i) Ø I N - - I N - (iii) (iv) (v) (vii) (viii) a) [3 points] For each of the eight configurations, draw in the magnetic dipole moment vector μ of the current loop and indicate whether the torque on the dipole due to the magnetic field is clockwise (CW), counterclockwise (CCW), or zero. In which two orientations will the loop experience the maximum magnitude of torque? [Hint: Use the…arrow_forwardPlease help with calculating the impusle, thanks! Having calculated the impact and rebound velocities of the ping pong ball and the tennis ball calculate the rebounding impulse: 1.Measure the weight of the balls and determine their mass. Tennis ball: 0.57 kg Ping Pong Ball: 0.00246 kg The impulse, I, is equal to the change in momentum, Pf-Pi. Note the sign change, i.e., going down is negative and up is positive. The unit for momentum is kg-m/s. The change is momentum, impulse, is often givens the equivalent unit of N-S, Newton-Secondarrow_forward
- 5. Three blocks, each with mass m, are connected by strings and are pulled to the right along the surface of a frictionless table with a constant force of magnitude F. The tensions in the strings connecting the masses are T1 and T2 as shown. m T1 T2 F m m How does the magnitude of tension T₁ compare to F? A) T₁ = F B) T₁ = (1/2)F C) T₁ = (1/3)F D) T₁ = 2F E) T₁ = 3Farrow_forwardUsing Coulombs Law, what is the magnitude of the electrical force between two protons located 1 meter apart from each other in Newtons?arrow_forwardCalculate the magnitude of the gravitational force between 2 protons located 1 meter apart from each other in Newtons using Newton's Law of Universal Gravitation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY