
Concept explainers
Interpretation: The part of Figure P21.1 that best describes the periodic trend in monatomic cation radii moving up or down a group or across a period in the periodic table is to be stated.
Concept introduction: On moving up to down in a group, the principal quantum number ‘n’ increases. Number of shells of electrons increases down the group. Therefore, the size of cation increases down the group.
To determine: The part of Figure P21.1 that best describes the periodic trend in monatomic cation radii moving up or down a group or across a period in the periodic table.
Answer to Problem 21.1VP
Solution
The part of Figure P21.1 that best describes the periodic trend in monatomic cation radii moving up or down a group or across a period in the periodic table is part (d).
Explanation of Solution
Explanation
The periodic trends in monatomic cation radii moving up or down a group or across a period in the periodic table are shown below.
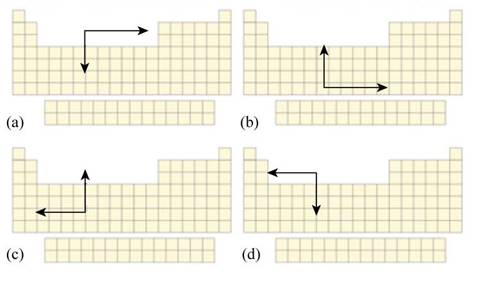
Figure 1
A cation is formed when a neutral atom loses an electron. When an electron is lost, the electron-electron repulsion in an atom decreases. Hence, the interaction between nucleus and valence electrons increases. Therefore, the size of cation is less than the corresponding atom
On moving up to down in a group, the principal quantum number ‘n’ increases. Number of shells of electrons increases down the group. Therefore, the size of cation increases down the group. On moving right to left, the principal quantum number remains the same but the effective nuclear charge of electron increases. The
The part that describes the trend in monatomic cation radii moving up or down a group or across a period in the periodic table is part (d) because in this part, cationic radii is increasing on moving down the group and on moving right to left in a period.
Conclusion
The part of Figure P21.1 that best describes the periodic trend in monatomic cation radii moving up or down a group or across a period in the periodic table is part (d).
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: The Science in Context (Fifth Edition)
- Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forward
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





