
a)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name is 3-bromo-4-methoxybenzoic acid.
Explanation of Solution
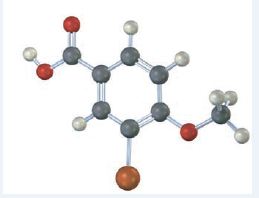
The compound represented by the model is
It is a benzoic acid derivative with a bromine atom on C3 and a methoxyl group on C4.
The IUPAC name of the compound represented by the model shown is 3-bromo-4-methoxybenzoic acid.
b)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
Explanation of Solution
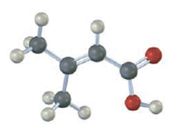
The compound represented by the model is
The compound is an unsaturated acid having a four carbon straight chain with a double bond between C2 and C3.
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
c)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
Explanation of Solution
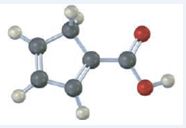
The compound represented by the model is
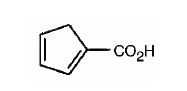
The compound has a cyclopentadiene ring to which a carboxyl group is attached. The double bonds in the diene are in between C1 & C2 and C3 & C4.
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
d)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Explanation of Solution
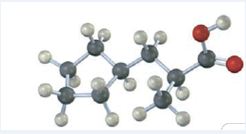
The compound represented by the model is
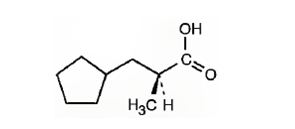
The compound is a carboxylic acid with a three carbon straight chin and has a methyl group attached to C2 and cyclopentyl group attached to C3. The compound is optically active as C2 is chiral. The groups of first highest, second highest and third highest groups when viewed away from the group of last priority are arranged in the anti-clockwise direction. Hence the compound has S stereochemistry.
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

