
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20.9, Problem 16P
Draw the products of each reaction.
a. 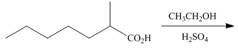 c.
c. 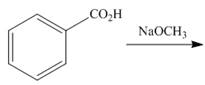
b. 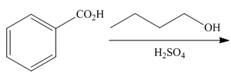 d.
d. 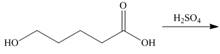
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write structural formulas for the major products by
doing addition reactions
1. You must add H2 as Pt is catalyst it does not take part in reactions
only speed up the process
H₂
CH2=CH-CH3
Pt
2. Add HCI break it into H and Cl
CH3
HCI
3. Add Br2 only CC14 is catalyst
CH3-CH=CH2
B12
CCl4
4. Add water to this and draw major product, H2SO4 is catalyst you have add
water H20 in both the reaction below
H₂SO4
CH3-CH=CH2
CH3
H2SO4/H₂O
CH3-C=CH2
reflux
?
Plan the synthesis of the following compound using the starting
material provided and any other reagents needed as long as
carbon based reagents have 3 carbons or less. Either the
retrosynthesis or the forward synthesis (mechanisms are not
required but will be graded if provided) will be accepted if all
necessary reagents and intermediates are shown (solvents and
temperature requirements are not needed unless specifically
involved in the reaction, i.e. DMSO in the Swern oxidation or
heat in the KMnO4 oxidation).
H
H
Hint These are benzene substitution reactions.
ALCI3 and UV light are catalyst no part in reactions and triangle A means
heating.
A. Add ethyl for Et in benzene ring alkylation reaction EtCl =
CH3CH2CL
1) EtC1 / AlCl3 / A
?
B: Add Br to benzene ring ( substitution)
2) Br₂ / uv light
?
C Add (CH3)2 CHCH2 in benzene ring ( substitution)
(CH3)2CHCH,C1 / AICI,
?
Chapter 20 Solutions
Organic Chemistry (6th Edition)
Ch. 20.1 - Prob. 1PCh. 20.2 - Draw the three possible resonance structures for...Ch. 20.2 - Prob. 3PCh. 20.3 - Give an IUPAC or common name for each compound. a....Ch. 20.3 - Problem 22.5 Draw the structure corresponding to...Ch. 20.4 - Problem 22.6 Explain why the boiling point of is...Ch. 20.7 - Prob. 11PCh. 20.7 - Prob. 12PCh. 20.8 - Prob. 13PCh. 20.9 - Problem 22.16 Draw the products of each reaction.
...
Ch. 20.9 - Prob. 17PCh. 20.9 - Problem 22.18 Draw a stepwise mechanism for the...Ch. 20.9 - Prob. 19PCh. 20.10 - Problem 22.20 Fenofibrate is a...Ch. 20.10 - Problem 22.21 What product is formed when the...Ch. 20 - Prob. 33PCh. 20 - 22.40 Give the IUPAC or common name for each...Ch. 20 - 22.41 Give the structure corresponding to each...Ch. 20 - Prob. 36PCh. 20 - 22.43 Explain why is a stronger acid and a weaker...Ch. 20 - (a) Propose an explanation for the difference in...Ch. 20 - Draw the product formed when phenylacetic acid is...Ch. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - Prob. 47PCh. 20 - Prob. 48PCh. 20 - 22.64 What carboxylic acid and alcohol are needed...Ch. 20 - Problem 22.65 Devise a synthesis of each compound...Ch. 20 - 22.70 What polyester or poly amide can be prepared...Ch. 20 - 22.71 What two monomers are needed to prepare each...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY