
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20.1, Problem 1P
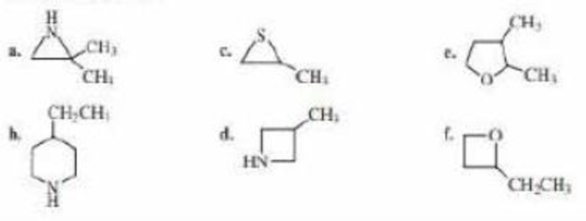
Name the following:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can I get some help drawing my arrows. I included what the final needs to look like
please help
(a)
(e)
O₂N.
(h)
21.8 Name the following compounds.
Br
(f)
Ph.
(c)
(d)
Br
(g)
NO₂
H
NH2
Br
mo. 0-0.
OMe
(i)
Chapter 20 Solutions
Organic Chemistry
Ch. 20.1 - Name the following:Ch. 20.2 - Prob. 2PCh. 20.2 - Prob. 3PCh. 20.3 - Draw the product of each of the following...Ch. 20.5 - Prob. 6PCh. 20.5 - When pyrrole is added to a dilute solution of...Ch. 20.5 - Explain why cyclopentadiene (pKa = 15) is more...Ch. 20.6 - Prob. 10PCh. 20.6 - How to the mechanisms of the following reactions...Ch. 20.6 - Prob. 12P
Ch. 20.6 - Rank the following compounds from easiest to...Ch. 20.7 - Prob. 14PCh. 20.7 - Prob. 15PCh. 20.7 - Prob. 16PCh. 20.7 - Prob. 17PCh. 20.7 - Prob. 18PCh. 20.7 - Prob. 19PCh. 20.7 - Prob. 20PCh. 20 - Name the following:Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Which of the following compounds is easier to...Ch. 20 - Rank the following compounds from most reactive to...Ch. 20 - One of the following compounds undergoes...Ch. 20 - Benzene undergoes electrophilic aromatic...Ch. 20 - The dipole moments of furan and tetrahydrofuran...Ch. 20 - Prob. 29PCh. 20 - Prob. 30PCh. 20 - The chemical shifts of the C-2 hydrogen in the...Ch. 20 - Explain why protonating aniline has a dramatic...Ch. 20 - Prob. 33PCh. 20 - Propose a mechanism for the following reaction:Ch. 20 - Prob. 35PCh. 20 - Propose a mechanism for the following reactions:Ch. 20 - Prob. 37PCh. 20 - a. Draw resonance contributors to show why...Ch. 20 - Prob. 39PCh. 20 - Pyrrole reacts with excess...Ch. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Organic chemists work with tetraphenylporphyrins...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY