
Concept explainers
Interpretation:
The structural formula for each of the given compounds is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
First identify the word root for the given compound.
The suffix used in the compound like –ane, ene, yne, ol, al and so on.
Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 28P
Solution:
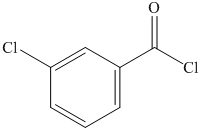
a) The structural formula of m-chlorobenzoyl chlorideis shown below.
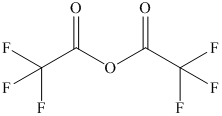
b) The structural formula of trifluoroacetic anhydride is shown below.
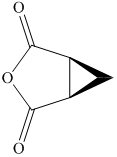
c) The structural formula of cis-
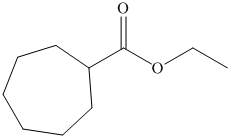
d) The structural formula of ethyl cycloheptanecarboxylateis shown below.
(e) The structural formula of
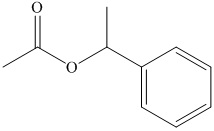
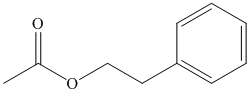
(f) The structural formula of
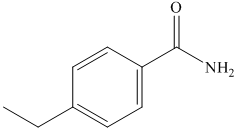
(g) The structural formula of p-ethylbenzamide is shown below.
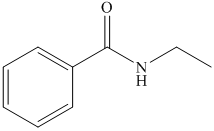
(h) The structural formula of N-ethylbenzamide is shown below.
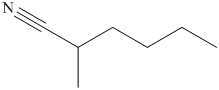
(i) The structural formula of
Explanation of Solution
a) m-Chlorobenzoyl chloride.
The name of compound suggests that chlorine group is present at meta position with respect to acyl group on benzene ring. The structure of compound is shown below.

b) Trifluoroacetic anhydride.
The name of the compound shows that anhydride group
group. The structure of compound is shown below.

c) cis-
The name of the compound indicates that two carboxylic groups are present on cyclopropane. Both carboxylic groups are present on the same plane. The structure of compound is shown below.

d) Ethyl cycloheptanecarboxylate
The name of the compound suggests that ester group is present in the structure. The cycloheptane ring is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(e)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(f)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The second carbon atom is bonded to benzene ring. The structure of compound is shown below.

(g) p-Ethylbenzamide
The name of compound suggests that ethyl group is present at para position with respect to amide group on benzene ring. The structure of compound is shown below.

(h) N-Ethylbenzamide
The name of compound suggests that ethyl group is bonded to amide group which further bonded to benzene ring. The structure of compound is shown below.

(i)
The name of the compound indicates that it contains hydrocarbon chain of six carbon atoms. On the second carbon atom methyl group is present and on the first carbon atom nitrile group is present. The structure of compound is shown below.

Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - Standalone book
- Draw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forward
- Draw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forwardSyntheses 22.35 Show how to convert toluene to these compounds. (a) -CH,Br (b) Br- -CH3 22.36 Show how to prepare each compound from 1-phenyl-1-propanone. 1-Phenyl-1-propanone ہتی. Br. (b) Br (racemic) 22.37 Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid. 22.38 Show reagents and conditions to bring about the following conversions. (a) 9 NH2 8 CO₂H NH2 CO₂Et (d) NO2 NH2 S NH₂ NO2 CHS CHarrow_forward
- ive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardCalculate the stoichiometric amount of CaCl2 needed to convert all of the CuSO4 into CuCl2.arrow_forwardH CH تنی Cl 1. NaCN, DMF 2. LIAIH4, ether H₂O pyridine N NH₂ 5 CH H 1 HNO, H₂SO 2. Nal NH2 Br Br HNO₂ CuCl H₂SO HCI CH3 H3C NN HSO KCN CuCN 1. HNO₂, H₂SO O₂N NH2 2. OH ཀ་ལས། །ས་ཅན་ :i་དེ་མ་མ་སེ་ NH₂ CH3 1. HNO₂, H₂SO4 2. H3PO₂ 1 HNO2, H2SO4 2. Nalarrow_forward
- ive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forwardIf a pharmacy chain sold 65 million 500-mg tablets of aspirin, how many US tons of aspirin does this represent? Report your answer to 2 significant figures.arrow_forwardHere are the options: reducing a monosaccharide a non reducing disaccharide amylopectin cellulose 1,4' beta- glycosidearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





