
Concept explainers
Thioesters
Thioesters have the general formula 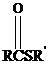 . They resemble their oxygen counterparts
. They resemble their oxygen counterparts 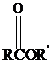
(oxoesters) in structure and reactivity more than other
chlorides, acid anhydrides, and amides. Thioesters can be prepared from thiols by reaction with acyl chlorides or acid anhydrides in much the same way as oxoesters are prepared from
alcohols.
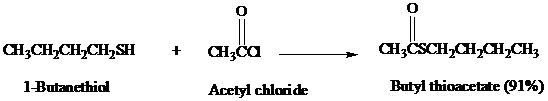
The preparation of thioesters by Fischer esterification is not very effective, however, because
the equilibrium is normally unfavorable. Under conditions in which ethanol is converted to ethyl
benzoate to the extent of
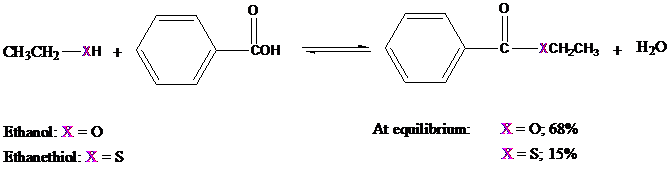
This, and numerous other observations, indicates that
Like chlorine, sulfur is a third-row element and does not act as an electron-pair donor to the carbonyl group as well as oxygen.
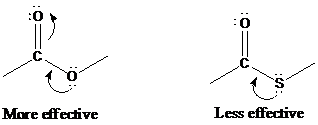
Thioesters and oxoesters are similar in their rates of nucleophilic acyl substitution, except with
amine nucleophiles for which thioesters are much more reactive. Many biological reactions involve nucleophilic acyl substitutions referred to as acyl transfer reactions. The thioester acetyl coenzyme A is an acetyl group donor to alcohols,
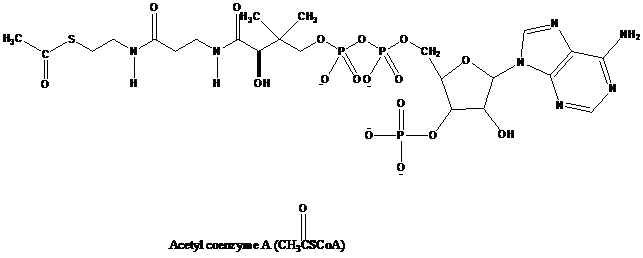 Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the
Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the

Acetylcholine is a neurotransmitter formed in nerve cells by the enzyme-catalyzed reaction
of choline with acetyl coenzyme A.
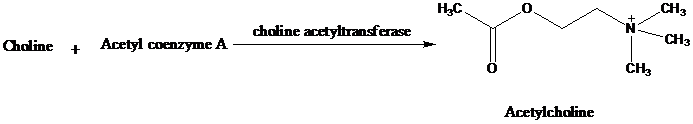
What is the most reasonable structure for choline?
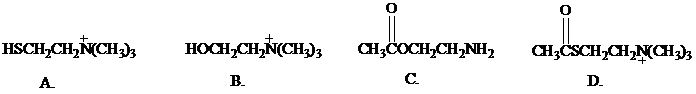
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry - Standalone book
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
- Using the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


