
DATA You are
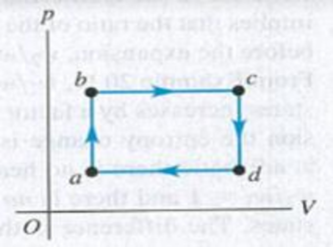
Figure P20.57
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
University Physics with Modern Physics (14th Edition)
Additional Science Textbook Solutions
An Introduction to Thermal Physics
Essential University Physics: Volume 2 (3rd Edition)
The Cosmic Perspective (8th Edition)
University Physics Volume 1
Tutorials in Introductory Physics
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
- In Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is 500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? Figure P19.22 (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?arrow_forwardFigure P21.37 shows a PV diagram for a gas that is compressed from Vi to Vf. Find the work done by the a. gas and b. environment during this process. Does energy enter the system or leave the system as a result of work? FIGURE P21.37arrow_forwardA 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? Find (c) Q, (d) Eint, and (e) W for the gas during this process.arrow_forward
- (a) Determine the work done on a gas that expands from i to f as indicated in Figure P19.16. (b) What If? How much work is done on the gas if it is compressed from f to i along the same path? Figure P19.16arrow_forwardIf a gas is compressed isothermally, which of the following statements is true? (a) Energy is transferred into the gas by heat. (b) No work is done on the gas. (c) The temperature of the gas increases. (d) The internal energy of the gas remains constant. (e) None of those statements is true.arrow_forwardA thermodynamic cycle is shown in Figure P21.34 for a gas in a piston. The system changes states along the path ABCA. a. What is the total work done by the gas during this cycle? b. How much heat is transferred? Does heat flow into or out of the system? Figure P21.34arrow_forward
- Air (a diatomic ideal gas) at 27.0C and atmospheric pressure is drawn into a bicycle pump (Figure P17.53) that has a cylinder with an inner diameter of 2.50 cm and length 50.0 cm. The downstroke adiabatically compresses the air, which reaches a gauge pressure of 8.00 105 Pa before entering the tire. We wish to investigate the temperature increase of the pump. (a) What is the initial volume of the air in the pump? (b) What is the number of moles of air in the pump? (c) What is the absolute pressure of the compressed air? (d) What is the volume of the compressed air? (e) What is the temperature of the compressed air? (f) What is the increase in internal energy of the gas during the compression? What If? The pump is made of steel that is 2.00 mm thick. Assume 4.00 cm of the cylinders length is allowed to come to thermal equilibrium with the air. (g) What is the volume of steel in this 4.00-cm length? (h) What is the mass of steel in this 4.00-cm length? (i) Assume the pump is compressed once. After the adiabatic expansion, conduction results in the energy increase in part (f) being shared between the gas and the 4.00-cm length of steel. What will be the increase in temperature of the steel after one compression? Figure P17.53arrow_forwardThe arrow OA in the PV diagram shown in Figure OQ22.11 represents a reversible adiabatic expansion of an ideal gas. The same sample of gas, starting from the same state O. now undergoes an adiabatic free expansion to the same final volume. What point on the diagram could represent the final state of the gas? (a) the same point A as for the reversible expansion (b) point B (c) point C (d) any of those choices (e) none of those choicesarrow_forwardOne mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forward
- In a cylinder of an automobile engine, immediately after combustion the gas is confined to a volume of 50.0 cm3 and has an initial pressure of 3.00 106 Pa. The piston moves outward to a final volume of 300 cm3, and the gas expands without energy transfer by heat, (a) What is the final pressure of the gas? (b) How much work is done by the gas in expanding?arrow_forwardA 1.00-mol sample of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate (a) the energy transferred to the gas by heat, (b) the increase in its internal energy, and (c) the work done on the gas.arrow_forwardYou have a particular interest in automobile engines, so you have secured a co-op position at an automobile company while you attend school. Your supervisor is helping you to learn about the operation of an internal combustion engine. She gives you the following assignment, related to a simulation of a new engine she is designing. A gas, beginning at PA = 1.00 atm, VA = 0.500 L, and TA = 27.0C, is compressed from point A on the PV diagram in Figure P19.31 (page 530) to point B. This represents the compression stroke in a fourcycle gasoline engine. At that point, 132 J of energy is delivered to the gas at constant volume, taking the gas to point C. This represents the transformation of potential energy in the gasoline to internal energy when the spark plug fires. Your supervisor tells you that the internal energy of a gas is proportional to temperature (as we shall find in Chapter 20), the internal energy of the gas at point A is 200 J, and she wants to know what the temperature of the gas is at point C. Figure P19.31arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning




