
Interpretation:
Whether an achiral or two equal enantiomeric products would be given has to be predicted in the product of the given reaction and explained with chair like transition state.
Concept Introduction:
Cope-rearrangement:
It is a pericyclic reaction that involves the redistribution of six electrons through the formation of a cyclic transition state from which a
Example with mechanism of cope-arrangement:
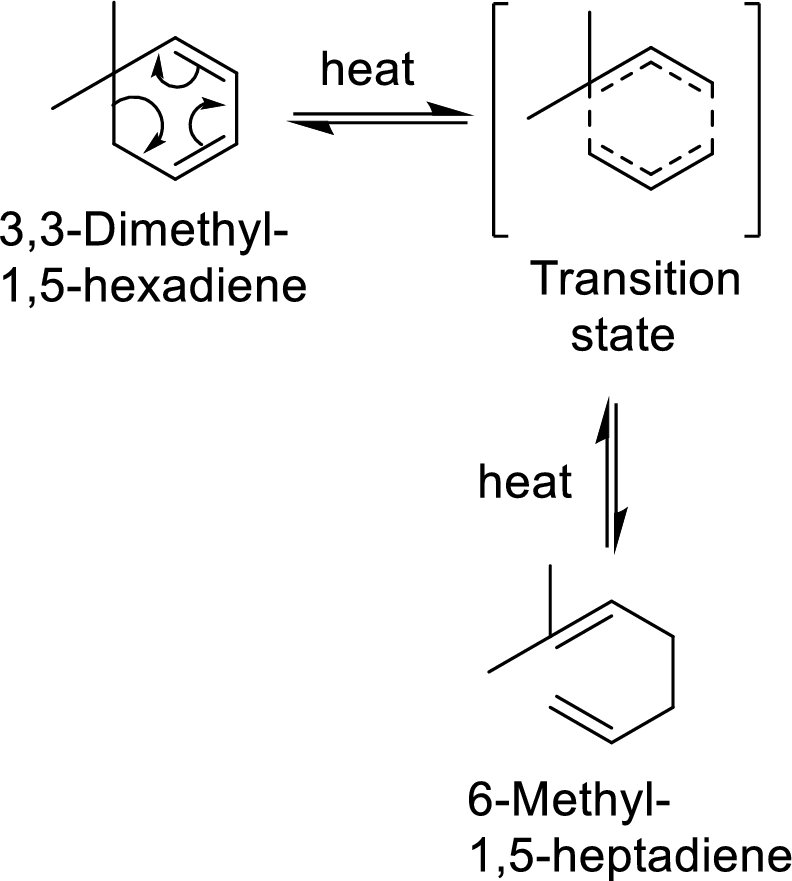
In this mechanism, two pi-bonds and one sigma bond of the reactant molecule has been rearranged and formed two new pi-bonds through a cyclic transition state.
Identification of cope-rearrangement in a
In the cope-rearrangement, the flow of electrons takes place between six bonds that are bonded as
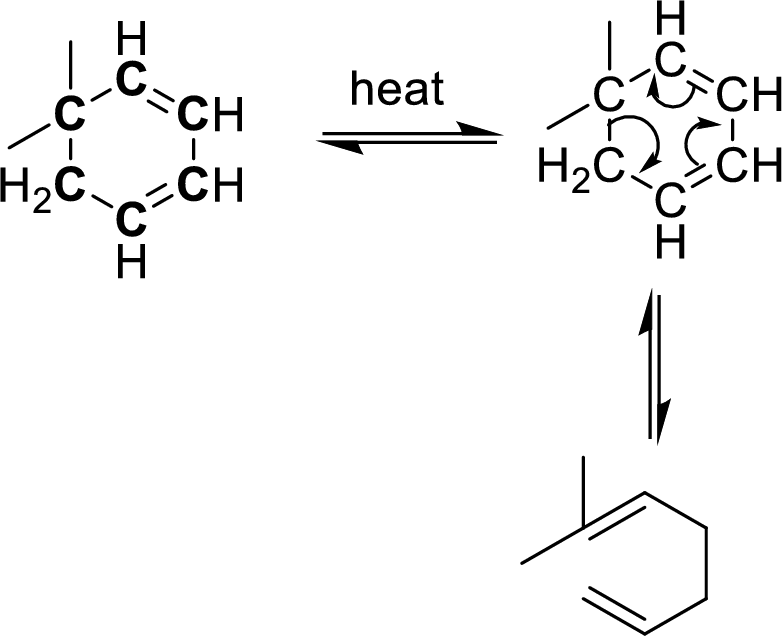
The carbon atoms that are involving in the cope-rearrangement are shown in bold.
Stereochemistry in a product formed:
- • In the product of a
chemical reaction , if a carbon atom has been attached with four different carbon atoms, then it is known as chiral carbon atom or stereocenter in the product. - • The bonds of the
functional groups because of which a new chiral carbon is supposed to form have to be represented in solid wedge bond and hashed-wedge bonds according to the particular enantiomer. - • Racemic mixture is the mixture of two enantiomers in equal proportions.
- • Enantiomers are non-superimposable mirror images.
- • Achiral product is the product in which there won’t be any chiral centre or stereocenter.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry, Loose-leaf Version
- Gas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forward
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

